|
 |
- Search
| Anesth Pain Med > Volume 17(2); 2022 > Article |
|
Abstract
Background
Local anesthetics systemic toxicity (LAST) is a grave complication of regional anesthesia that usually occurs immediately after local anesthetics injection. Here, we report on rare late-onset toxicity cases after supraclavicular brachial plexus blocks.
Case
Two patients underwent surgery for radius fractures. We used lidocaine 100 mg and ropivacaine 150 mg for blocking and infused dexmedetomidine for intraoperative sedation. The 63-year-old male patient’s blood pressure dropped to 87/60 mmHg after 3 h 15 min after blocking. Ventricular fibrillation occurred 10 min later. After five defibrillations, electrocardiography showed ventricular tachycardia that was normalized through one cardioversion. The 54-year-old female patient’s heart rate decreased to 35 beats/min 2 h 30 min after blocking. Her vital signs returned to normal after administering atropine, ephedrine, epinephrine, and lipid emulsion.
Anesthesiologists must be aware of local anesthetics systemic toxicity (LAST), which is a fatal complication affecting the central nervous system and cardiovascular system that can occur during local anesthesia [1]. LAST occurs 1.8 times per 1,000 nerve blocks and most cases occur shortly after local anesthetic injection (< 10 min: 53%, 11-60 min: 19%) [2]. Here, we report cases of suspected late-onset LAST, which is relatively rare, after a single injection of local anesthetics during supraclavicular brachial plexus block (BPB).
A 64-year-old male (170 cm, 88 kg) with a history of hypertension and hyperlipidemia (American Society of Anesthesiologists physical status 2) visited our hospital with a radius fracture. The patient’s blood pressure was well controlled and there were no abnormalities in his preoperative electrocardiogram (EKG), blood tests, or chest radiograph images. The patient was scheduled to undergo open reduction and internal fixation surgery using supraclavicular BPB. The patient was moved to the block room to monitor his non-invasive blood pressure (NIBP), pulse oximetry, and EKG status.
The location of the brachial plexus was identified with an EPIQ 7 3.0-12.0 MHz 38 mm linear transducer ultrasound device (Philps, Netherlands) and a 22 gauge 50 mm Stimuplex Ultra 360 needle (B. Braun, Germany) was introduced into the target area using an in-plane technique (Fig. 1). The target nerve was identified by stimulating it with a current of 0.4 mA and observing the corresponding muscle twitching. To prevent intravascular injection, we ensured that blood was not aspirated through several regurgitations during the procedure. We used a mixed solution of 1% lidocaine 10 ml (100 mg) (Daihan Pharm, Korea) and 0.75% ropivacaine 20 ml (150 mg) (Mitsubishi Tanabe Pharma Korea, Korea) as local anesthetics. We checked whether there was a change in mental status through verbal communication during and immediately after the procedure. The patient’s vital signs immediately after anesthesia were stable (NIBP: 147/97 mmHg, heart rate: 88 beats/min). We confirmed sensory blockage by asking the patient to compare the sensation in the arm that received the anesthetic with that of the contralateral arm and confirmed the motor blockage by checking that the patient could not lift his anesthetized arm over his head. Dexmedetomidine (Pfizer Pharmaceuticals Korea, Korea) infusion was started 5 min after local anesthetic injection for intraoperative sedation in the operating room. It was infused at 480 μg/h for 10 min for loading then at 48 μg/hr. We infused dexmedetomidine for 1 h 50 min (total dose: 160 μg, 40 ml) and the operation took 2 h 30 min. After the cessation of dexmedetomidine infusion 1 h 55 min after local anesthetic administration, the EKG monitor showed premature ventricular contraction bigeminy, but it quickly disappeared and the patient’s vital signs did not show any abnormalities (NIBP: 115/77 mmHg, heart rate: 62 beats/min). We checked that there was no change in the patient’s mental status by communicating with him.
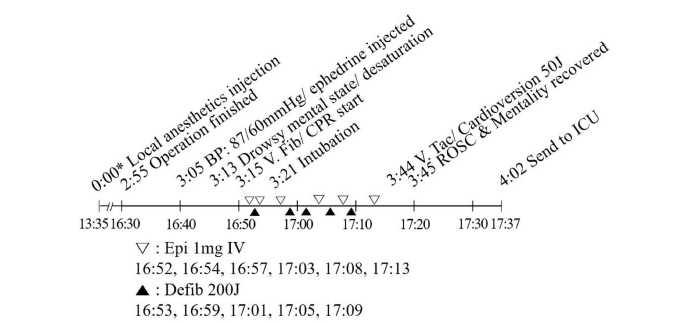
The patient was moved to the post-anesthesia care unit (PACU) and monitored by standard monitoring procedures after 3 h 5 min after local anesthetic administration. Ten min after being moved to the PACU, which was 1 h 10 min after dexmedetomidine cessation, the patient’s NIBP suddenly decreased to 87/60 mmHg and his heart rate was 61 beats/min. We administered ephedrine 5 mg intravenously and his NIBP slightly increased to 90/62 mmHg and his heart rate was 56 beats/min. However, after eight min, the patient seemed drowsy and did not respond to his name. As his peripheral oxygen saturation decreased to 87%, we assisted the patient’s breathing with a Jackson-Rees circuit (King Systems, USA). Two min after his mental state changed, the EKG monitor showed ventricular fibrillation (Fig. 2A) and we started chest compressions. We defibrillated the patient immediately and the EKG returned to a normal rhythm and maintained this pattern for a while. However, ventricular fibrillation occurred again. The patient’s EKG changed to ventricular tachycardia (Fig. 2B) after five defibrillations (200 J) and returned to a normal sinus rhythm through one synchronized cardioversion (50 J). We intubated the patient during cardio-pulmonary resuscitation and administered epinephrine 1 mg intravenously six times. We confirmed that the patient’s mental status had recovered after spontaneous circulation resumed 30 min after the cardiac arrest occurred (Fig. 3). Afterward, the patient was transferred to the intensive care unit and norepinephrine was infused at 0.2 μg/kg/min to maintain BP. Immediately after the patient was moved to the intensive care unit, we took 12 leads EKG that showed QT prolongation (Fig. 2C). Pulmonary edema was found on the chest radiograph and chest computed tomography (CT). We decided to maintain mechanical ventilation and started sedation with dexmedetomidine. No abnormal findings were found in the blood tests, EKG, and chest CT but Holter’s test showed multiple premature ventricular contractions (3,192 times/24 h). One day after surgery, chest X-ray images did not show pulmonary edema and we performed extubation. Five days after surgery, the patient was moved to the general ward and was discharged eight days after surgery without any sequelae.
A 54-year-old female (150 cm, 70 kg) without any underlying diseases (American Society of Anesthesiologists physical status 1) visited the hospital with a radius fracture. The patient was scheduled to undergo open reduction and internal fixation surgery using supraclavicular BPB. There were no abnormalities in her preoperative exams.
Anesthesia was performed in the same manner as in the previous case using the same local anesthetic drug and dose. There were no abnormalities in the patient’s condition during the procedure. Dexmedetomidine was administrated for intraoperative sedation. After 1 h 50 min of surgery, the patient was transferred to the PACU.
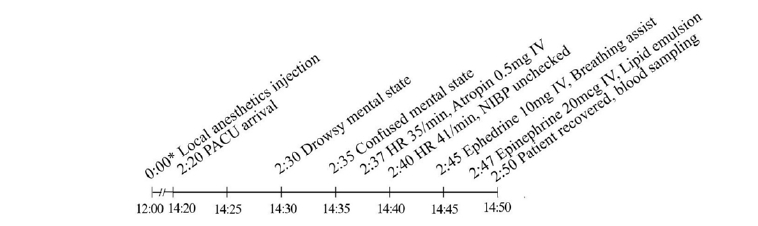
Two hours 20 min after local anesthetic administration, the patient was moved to the PACU and monitored by standard monitoring. After 10 min, the patient showed a drowsy mental state. Seven min later, her NIBP was maintained at 98/57 mmHg but her heart rate decreased to 35 beats/min. To resolve bradycardia, we administered atropine 0.5 mg intravenously. Her heart rate increased slightly to 41 beats/min, but her NIBP could not be measured. Hypotension and bradycardia were treated with two intravenous administrations of ephedrine 10 mg. The patient seemed confused, so we assisted her breathing with a Jackson-Rees circuit (King Systems, USA). Then, suspecting LAST, we injected 20% lipid emulsion (LE) (Fresinius Kabi Korea, Korea) 100 ml as a bolus over three min followed by infusion at a rate of 17.5 ml/min and injected epinephrine 20 μg. After injection of epinephrine and LE, the patient’s NIBP and heart rates increased and her mental state returned to normal (NIBP: 193/97 mmHg, heart rate: 121 beats/min) (Fig. 4). The LE infusion was terminated 10 min after the patient's vital signs were confirmed to be stable. The patient stayed in the PACU for about 1 h and was then transferred to the general ward after she was confirmed to have made a full recovery. Unlike in the previous case, a blood sample was taken from her radial artery to measure the concentration of ropivacaine, which was determined to be 1.1 μg/ml.
Written informed consent to publish these cases was obtained from both patients.
This case report was about a 64-year-old male and a 54-year-old female who underwent supraclavicular BPB for surgery to treat radius fractures and developed cardiovascular symptoms 2-3 h after local anesthetic administration.
For these cases, symptoms occurred long after the injection of local anesthetics, so we did not initially suspect LAST. It usually occurs shortly after the injection of local anesthetics and is usually caused by unintended direct intravascular injection. When toxicity is delayed, it is caused by infiltrated anesthetics being absorbed through the surrounding tissues [3]. More than 70% of LAST cases occurred within 1 h of local anesthetic administration and those that occurred after 1 h mainly occurred during continuous infusion of local anesthetics [4].
Rarely, cases of late-onset LAST that occurred after a single local anesthetic injection during BPB have been reported. Oh et al. [5] reported LAST that occurred 1 h after interscalene-axillary BPB single injection. They injected 0.75% ropivacaine 12 ml (90 mg) into the interscalene brachial plexus and then injected a mixed solution of 0.75% ropivacaine 8 ml (60 mg), 2% mepivacaine 20ml (400 mg), and normal saline 20 ml to the axillary brachial plexus. They additionally injected 1% lidocaine 5 ml (50 mg) to block the intercostobrachial nerve. The patient experienced seizures 1 h after injection of local anesthetics but no cardiovascular system symptoms. İnceöz et al. [6] reported LAST that occurred 7 h after infraclavicular BPB single injection. However, they used bupivacaine and prilocaine, which have longer half-lives than the local anesthetics used in our cases and the total amount of drugs used was also large (0.5% bupivacaine 11.25 ml, 56.25 mg + 2% prilocaine 11.25 ml, 225 mg). In addition, unlike our first case, cardiac arrest did not occur in their case.
There are no well-established diagnostic criteria for LAST. LAST is diagnosed through clinical symptoms. Differential diagnoses of LAST include anaphylaxis, anxiety, methemoglobinemia, and reaction to the vasoconstrictor. These complications have similar presentations to LAST’s clinical features, making differential diagnosis difficult. If possible, a blood test should be used to measure the concentration of local anesthetics [7]. In the first case, we could not measure the concentration of the local anesthetic, but in the second case, we took a blood sample to measure the concentration of the local anesthetics while administering an LE. We collected blood samples from the radial artery and confirmed the plasma concentration of free-form ropivacaine to be 1.1 μg/ml. The concentration was measured 2 h 50 min after injection of local anesthetics and 3 min after LE injection. The toxic threshold for ropivacaine has not been clearly established, so it may be challenging to ascertain whether the concentration of 1.1 μg/ml caused LAST or not. Knudsen et al. [8] found that ropivacaine toxicity occurred at concentrations of 0.34-0.85 μg/ml (arterial blood, free form) and Scott et al. [9] found ropivacaine toxicity at concentrations of 1-2 μg/ml (venous blood). Therefore, it is possible ropivacaine induced LAST in our cases. It is worth mentioning that concentrations differ vastly according to whether the blood sample was taken from an artery or vein and whether it was free-form or bound-form, so toxicity concentrations should be interpreted carefully.
We also used lidocaine but we could not measure its concentration due to technical issues. Lidocaine may aggravate LAST, but it has a shorter half-life than ropivacaine. A mixture of local anesthetics is used to achieve anesthesia sooner and reduce toxicity. However, there is some controversy about accelerating anesthesia onset [10] and whether the anesthetics’ toxicities are additive [11]. Therefore, it would be safer to assume that anesthetics’ toxicities are additive.
Although LAST likely occurred in these two cases, other factors might have worsened its clinical course. The first factor is that dexmedetomidine can cause bradycardia, hypotension, and even cardiac arrest as side effects [12]. Severe bradycardia and even cardiac arrest have been reported when dexmedetomidine is combined with other agents, such as lidocaine [13]. These symptoms were observed in both our cases. The second factor is that arm slings could have caused carotid sinus hypersensitivity. When the carotid sinus baroreceptor is compressed, it induces parasympathetic activation, which leads to hypotension and bradycardia. In severe cases, it can progress to syncope or cardiac arrest. Canbora et al. [14] reported carotid sinus hypersensitivity due to arm sling use in a 56-year-old female PACU patient who had undergone shoulder surgery. Both patients in our cases were wearing arm slings while sedated, so the weight of the anesthetized arm may have directly compressed the carotid sinus.
The use of ultrasound reduces most of the rapid-onset LAST caused by the direct intravascular injection of local anesthetics. However, it still occurs, so physicians must be vigilant about it and determine whether any of the following risk factors are present: Previous local anesthesia experience; being young or old because newborns, young children, and elderly patients have less lean muscle mass than other patients; having a low total body mass; having a systemic, cardiac, renal, hepatic, or metabolic disease or protein binding abnormality; being pregnant; and having low plasma binding protein or albumin levels, which is particularly common in infants. Anesthetic characteristics and dosages should be carefully analyzed and the injection site should be carefully planned. Ultrasound-guided nerve blockades, incremental injections with frequent aspiration, and using pharmacological markers, such as epinephrine can help prevent LAST [1,3,15].
If LAST occurs despite all these measures, certain measures need to be carefully taken to improve patient outcomes. According to the American Society of Regional Anesthesia and Pain Medicine, airway maintenance and oxygenation are prioritized in treatment. If seizures occur, benzodiazepine should be administered. Treatment of arrhythmia and hypotension that occurs as the result of LAST is different from their treatment as part of standard advanced cardiac life support. Epinephrine doses should start at less than 1 μg/kg. The American Society of Regional Anesthesia and Pain Medicine also recommends the early administration of LE. Patients weighing more than 70 kg should be administered a maximum of 100 ml bolus over 2-3 min then receive an infusion of up to 250 ml for 15-20 min. Patients weighing less than 70 kg should receive an injection of a maximum of 1.5 ml/kg bolus over 2-3 min followed by an infusion of up to 0.25 ml/kg/min [15].
In conclusion, physicians should be aware that LAST may occur long after a single injection of local anesthetics and should consider factors that may adversely affect its course, such as the use of dexmedetomidine and arm slings.
Fig. 1.
Ultrasonography image of supraclavicular brachial plexus with color doppler. BP: brachial plexus, SA: subclavian artery.
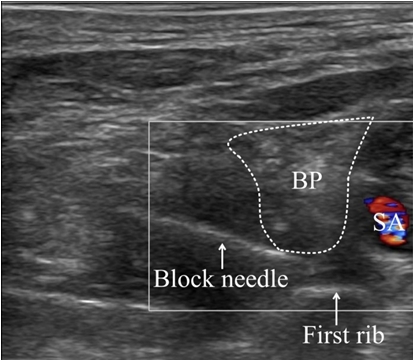
Fig. 2.
Electrocardiography of the patient. (A) Ventricular fibrillation. (B) Ventricular tachycardia. (C) QT prolongation: heart rate-corrected QT interval = 484 milliseconds.
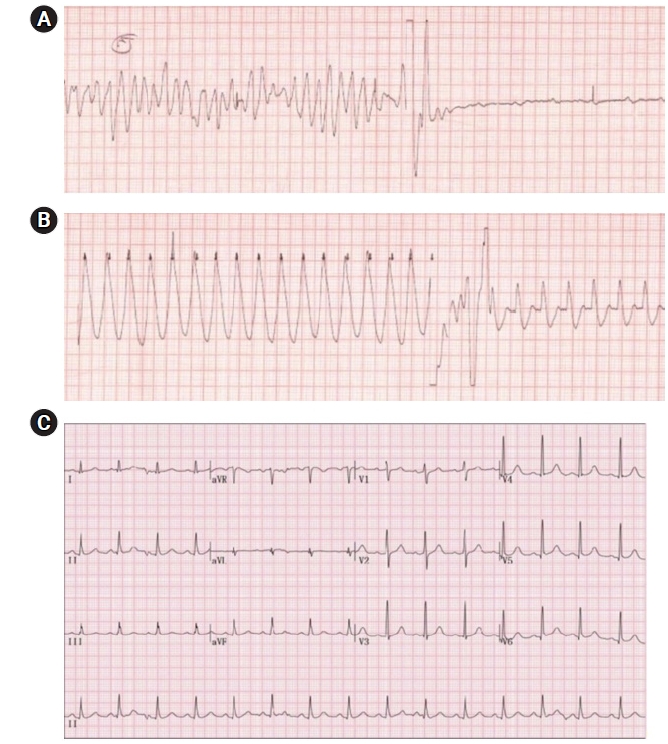
Fig. 3.
Timeline of the first case. BP: blood pressure, CPR: cardiopulmonary resuscitation, Defib: defibrillation, Epi: epinephrine, ICU: intensive care unit, IV: intravenously, ROSC: return of spontaneous circulation, V. Fib: ventricular fibrillation, V. Tac: ventricular tachycardia. *Indicates time after the local anesthetics injection.
REFERENCES
1. El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth 2018; 11: 35-44.



2. Macfarlane AJR, Gitman M, Bornstein KJ, El-Boghdadly K, Weinberg G. Updates in our understanding of local anaesthetic systemic toxicity: a narrative review. Anaesthesia 2021; 76 Suppl 1: 27-39.


3. Gitman M, Fettiplace MR, Weinberg GL, Neal JM, Barrington MJ. Local anesthetic systemic toxicity: a narrative literature review and clinical update on prevention, diagnosis, and management. Plast Reconstr Surg 2019; 144: 783-95.


4. Gitman M, Barrington MJ. Local anesthetic systemic toxicity: a review of recent case reports and registries. Reg Anesth Pain Med 2018; 43: 124-30.

5. Oh S, Chung J, Lee SM, Chung K, Kwon K. Late onset of systemic toxicity of local anesthetics in brachial plexus block: a case report. Anesth Pain Med 2012; 7: 372-4.
6. İnceöz H, Tutal ZB, Babayiğit M, Kepek A, Horasanlı E. Late local anaesthetic toxicity after infraclavicular block procedure. Turk J Anaesthesiol Reanim 2015; 43: 199-201.



7. Lui KC, Chow YF. Safe use of local anaesthetics: prevention and management of systemic toxicity. Hong Kong Med J 2010; 16: 470-5.

8. Knudsen K, Beckman Suurküla M, Blomberg S, Sjövall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth 1997; 78: 507-14.


9. Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg 1989; 69: 563-9.


10. Sepehripour S, Dheansa BS. Is there an advantage in onset of action with mixing lignocaine and bupivacaine? J Plast Reconstr Aesthet Surg 2017; 70: 1782.

11. Cuvillon P, Nouvellon E, Ripart J, Boyer JC, Dehour L, Mahamat A, et al. A comparison of the pharmacodynamics and pharmacokinetics of bupivacaine, ropivacaine (with epinephrine) and their equal volume mixtures with lidocaine used for femoral and sciatic nerve blocks: a double-blind randomized study. Anesth Analg 2009; 108: 641-9.


12. Bharati S, Pal A, Biswas C, Biswas R. Incidence of cardiac arrest increases with the indiscriminate use of dexmedetomidine: a case series and review of published case reports. Acta Anaesthesiol Taiwan 2011; 49: 165-7.


13. Beloeil H, Garot M, Lebuffe G, Gerbaud A, Bila J, Cuvillon P, et al. POFA Study Group; SFAR Research Network. Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery: the postoperative and opioid-free anesthesia (POFA) randomized clinical trial. Anesthesiology 2021; 134: 541-51.

- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others