Tube or tubeless: an anesthetic strategy for upper airway surgery
Article information
Abstract
Since the patient’s airway is shared between an anesthesiologist and a surgeon, airway management during upper airway surgery can be challenging. Beyond the conventional method of general anesthesia, high-flow nasal oxygenation (HFNO) has recently been used as a key technique for tubeless anesthesia. HFNO provides humidified, heated oxygen up to 70 L/min, which promises improved oxygenation and ventilation, allowing for prolonged apneic oxygenation. In previous physiological and clinical studies, HFNO has been demonstrated that tubeless anesthesia safely provide an uninterrupted surgical field during laryngeal surgeries. Although tubeless anesthesia remains uncommon, it can be a good alternative to conventional anesthesia if an anesthesiologist and a surgeon select appropriate patients together with sufficient experience. A safe strategy for tubeless anesthesia, along with appropriate backup plans, including endotracheal intubation and high-frequency jet ventilation, should be considered for upper airway surgery.
INTRODUCTION
Despite the concept deeming far from new, tubeless anesthesia has recently been witnessed more often during operations. With the ongoing trend of minimally invasive surgery backed by technological advances, it seems inevitable that anesthetic management should embrace these advancements as well. Beyond the early concept of apneic oxygenation, the advent of high-flow nasal oxygenation (HFNO) shows how technical advances in anesthesiology can be efficiently applied during surgery [1-5].
Since the patient’s airway is shared between the anesthesiologist and surgeon, upper airway surgery is a challenging task for both. By providing a tubeless surgical field during laryngeal surgery, HFNO has demonstrated feasibility as well as safety through physiological and clinical studies [6-14].
This review aims to provide a current overview of tubeless anesthesia and an appropriate anesthetic strategy for upper airway surgery.
THE CONVENTIONAL ANESTHESIA FOR UPPER AIRWAY SURGERY
Endotracheal intubation with a narrow tube has been considered the conventional method for laryngeal microsurgery (LMS) under general anesthesia and neuromuscular blockade. Under general anesthesia with endotracheal intubation, most anesthesiologists feel relieved that patient safety is established by securing the airway and controlling the ventilation. However, there are still potential risks of inadvertent airway injury during intubation and higher airway pressure due to the narrow endotracheal tube. In addition, intubated anesthesia may contribute to inadequate surgical exposure of posterior laryngeal lesions and a narrow working space in upper airway surgery.
For sufficient exposure of the periglottic area, the endotracheal tube must be extubated and reintubated intermittently during the procedure, when necessary. This inevitable apneic period can cause desaturation and hypercarbia, which can be fatal, particularly in patients with poorly compensated cardiopulmonary function. Furthermore, the durations of surgery and anesthesia are usually prolonged.
To improve the surgical conditions of the upper airway, high-frequency jet ventilation (HFJV) has been used since the 1980s, where high-capacity humidified oxygen-mixed air is regularly injected into a thin plastic cannula [15,16]. Compared to endotracheal intubation, the HFJV provides the surgeon with superior accessibility to the posterior part of the larynx with a wider view [17]. However, the technique has several disadvantages. Inappropriate airway pressure monitoring or insufficient expiratory airflow increases the risk of pulmonary barotrauma, manifesting as pneumothorax, hypoxemia, or hypercapnia. Moreover, because small tidal volumes are applied at a high physiological rate through a cannula, continuous adjustment is required to appropriately position the airflow during the entire surgical procedure [18]. Because of these disadvantages, HFJV is not widely used in upper airway surgery, despite its benefits as an alternative ventilatory support.
THE RATIONALE OF TUBELESS ANESTHESIA FOR UPPER AIRWAY SURGERY
Before the concept of HFNO, conventional anesthetic management was considered optimal for upper airway surgery, although anesthesiologists were discontent with manipulating the endotracheal tubes following the inevitable apneic periods. Apneic oxygenation is the administration of oxygen with a nasal prong or nasopharyngeal catheter during the apneic period and has been evaluated in patients undergoing general anesthesia since the early 20th century [19,20]. It revealed prolongation of the time to desaturation and lower the incidence of hypoxemia during endotracheal intubation and short laryngeal surgeries [1,21,22]. However, it is not an alternative to conventional anesthesia because of the rapid increase in carbon dioxide (CO2) and the subsequent decrease in pH [23].
Introduction of HFNO
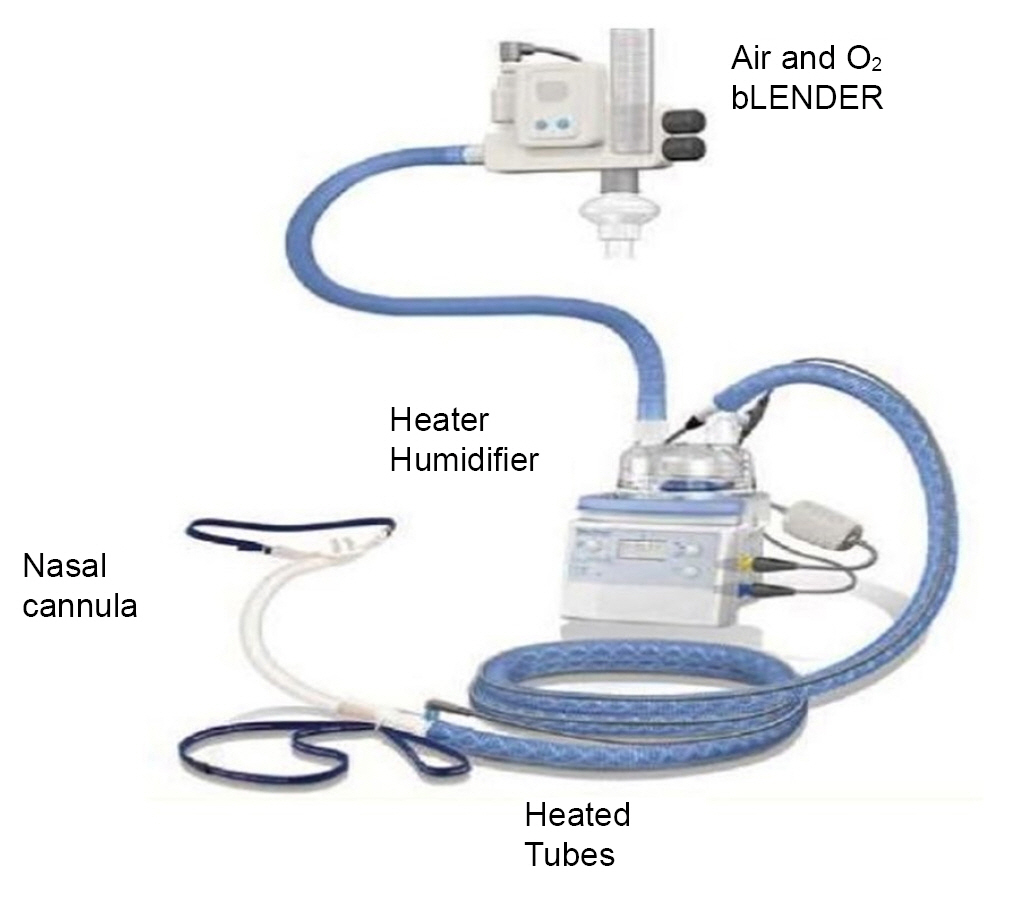
Transnasal humidified rapid-insufflation ventilatory exchange is a physiological term that implements HFNO using a nasal cannula. HFNO can provide efficient and safe apnea oxygenation. The most commonly used commercial device is the Optiflow™ system (Fisher & Paykel Healthcare), and it consists of an air/oxygen blender and flow meter, a heated humidifier, and wide-bore nasal prongs (Fig. 1) [24]. An air/oxygen blender connected to the circuit enables the anesthesiologist to precisely titrate the fractional inspired oxygen (FiO2) up to 1.0 at a high flow rate of up to 70 L/min. Despite the high flow rate, HFNO prevents dryness in the nasal cavity or discomfort by providing adequately heated (37℃) and humidified (44 mgH2O/L) gas [25].
Physiologic effects of HFNO
HFNO offers distinct advantages over other oxygenation methods because of its prominent positive effects on respiratory physiology. First, the continuous high flow of 100% oxygen causes denitrogenation and washout of the anatomical dead space [26,27]. During apnea, alveolar oxygen absorption exceeds CO2 production because of relative differences in blood solubility, generating a negative pressure gradient that favors the bulk flow of gas from the anatomical dead space into the alveoli, a phenomenon called aventilatory mass flow [28]. Increased alveolar ventilation flushes out expired CO2, thereby reducing the rate of CO2 accumulation and dead space rebreathing. Thus, HFNO therapy reduces the risk of hypercapnia in an apneic patient [29]. Secondly, HFNO generates a low level of continuous positive airway pressure (PEEP) of 2.7–7.4 cmH2O. The degree of PEEP depends on the flow rate, geometry of the upper airway, and breathing through the nose or mouth [8]. PEEP generated through HFNO assists in alveolar recruitment, preventing atelectasis and reducing shunting, thereby improving oxygenation. Furthermore, a heated and humidified air supply reduces breathing work, thereby reducing the energy requirements associated with gas conditioning. It also prevents against drying of the nasopharyngeal and tracheobronchial mucosa and improves mucociliary clearance with improving patient comfort [3,8,30].
HFNO in upper airway surgery
Owing to these physiological effects, HFNO has generated interest as a tubeless anesthetic for upper airway surgeries.
A number of prospective observational studies and case reports demonstrated the feasibility of HFNO as an alternative to conventional ventilation during laryngoscopic surgery [4,8-14,31-34]. Table 1 shows the characteristics and outcomes of the main reports on tubeless anesthesia in adult patients undergoing upper airway surgeries. In 2015, Patel and Nouraei [10] reported the benefits of tubeless anesthesia using HFNO in 25 patients with difficult airways who underwent hypopharyngeal or laryngotracheal surgery for the first time. All patients showed an extended median apneic time of 14 min without arterial desaturation < 90%. Notably, one patient showed an apneic time of 65 min, which allowed pharyngolaryngeal surgery to be completed without complications. Lyons and Callaghan [9] and Maupeu et al. [31] reported on the effects of tubeless laryngeal or tracheal surgery under apneic conditions with HFNO for sufficient gas exchange. The median apneic times were 19 and 27 min, respectively, ensuring adequate oxygenation and ventilation. Even still not common, emerging evidence has suggested that tubeless anesthesia with HFNO is effective in oxygenation and ventilation in most patients undergoing upper airway surgery.
IMPACT OF TUBELESS ANESTHESIA ON PATIENT OUTCOME
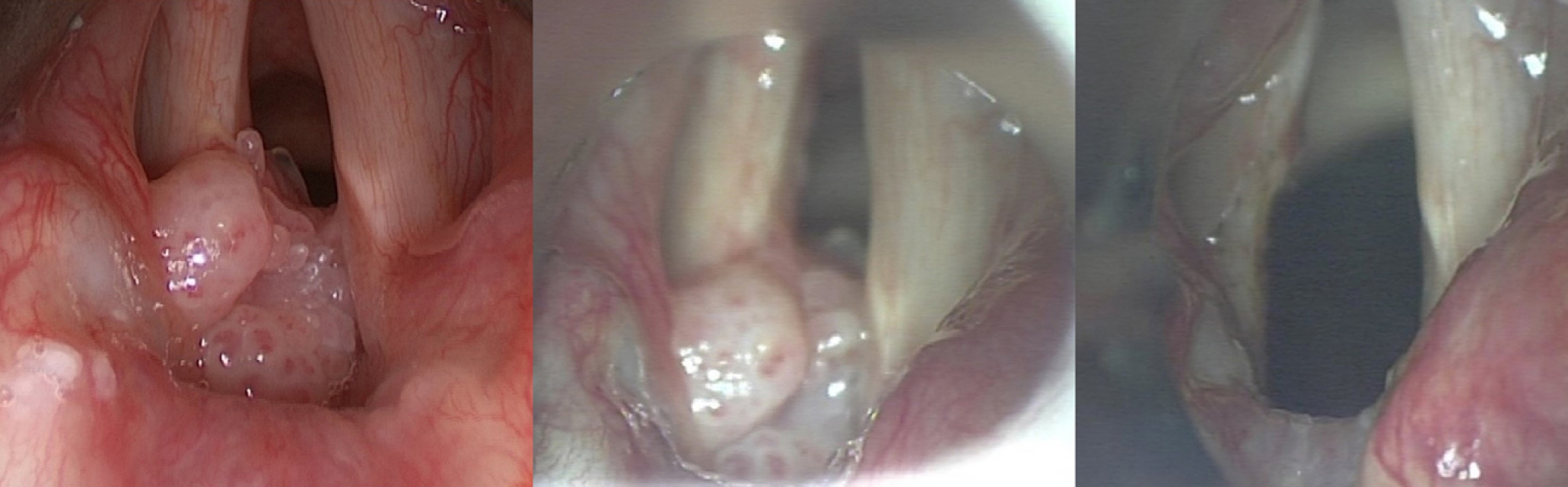
The most prominent benefits of tubeless anesthesia are improved operative outcomes and reduced time to laryngoscopic suspension and surgery by providing a better view of an uninterrupted surgical field (Fig. 2) [6,7,14,32]. Furthermore, postoperative patient comfort, as assessed by nausea, vomiting, and agitation, was acceptable because tubeless anesthesia is maintained only by total intravenous anesthesia [7,30,32]. These advantages allow tubeless anesthesia to be frequently used in the field of otorhinolaryngology for various upper airway surgeries ranging from microlaryngeal excision to surgery for subglottic stenosis. Among the favorable surgery-related and patient-related outcomes of HFNO, the most noteworthy are the excellent oxygenation and ventilation by CO2 clearance.
Oxygenation
Through the mass flow of oxygen, HFNO increases gas transfer and lung volume, which improves oxygenation during the procedure. Several studies reported comparable oxygenation on tubeless anesthesia using HFNO [8,10-12,30,34-36]. Min et al. [11] conducted a randomized controlled clinical study to compare the efficacy of HFNO and endotracheal intubation during LMS. This study revealed comparable oxygenation with HFNO and endotracheal intubation, demonstrating the feasibility of HFNO in upper airway surgery. In addition, the apnea time was up to 55 min in the HFNO group without complications. In addition, To et al. [12] reported the successful use of HFNO in 17 patients with subglottic stenosis who underwent balloon dilatation. The median apnea time to preserve appropriate oxygenation was 18 min. A retrospective study revealed excellent oxygenation with HFNO, which showed a low rate of oxygen desaturation, requiring jet ventilation rescue in patients with subglottic stenosis [37]. In this study, the median apnea time was > 40 min.
CO2 clearance
According to previous case reports and clinical studies, the rate of CO2 accumulation, rather than the oxygenation level, can limit the application of HFNO. Although HFNO reduces the rate of CO2 accumulation compared to apneic oxygenation [8,38], tubeless anesthesia is acceptable only for short laryngeal operations. The rate of CO2 increase during tubeless anesthesia using HFNO remains to be elucidated, as it is related to anesthetic techniques and methods of analysis. An experimental study using laboratory airway models demonstrated CO2 clearance differences according to the oxygen flow rate [39]. The mean CO2 clearance increased from 0.29 ml/min to 1.13 ml/min as the flow rate increased from 20 to 70 L/min. In clinical studies, EtCO2 was reported to increase 1.1 mmHg/min in patients with spontaneous ventilation, while peripheral venous CO2 being 1.6 mmHg/min and PaCO2 being 1.8 mmHg/min in apneic patients [9,10]. Although CO2 accumulation was nonlinear and leveled off over time, the most critical factor for discontinuing tubeless anesthesia is hypercarbia, which results in acidosis. Min et al. [11] demonstrated the best cutoff point for a safe apneic period of 28 min using HFNO, which may cause significant respiratory acidosis beyond this period, which is consistent with the results of other clinical studies. However, respiratory acidosis was resolved quickly by rescue endotracheal intubation or jet ventilation in previous studies, which supports the use of HFNO during short upper airway surgery with cautious monitoring and standby rescue methods. Among the reports and articles so far, no significant complications of tubeless anesthesia using HFNO have been reported in upper airway surgery. Potential complications can be prevented by closely monitoring oxygenation and ventilation.
STRATEGY OF TUBELESS ANESTHESIA FOR UPPER AIRWAY SURGERY
Patient selection
Surgical concerns regarding adequate access and visualization, as well as anesthetic concerns regarding oxygenation and ventilation, should be balanced when applying HFNO in upper airway surgery. In addition to the indications (patent airway and short laryngeal surgery) and contraindications (skull base defect, increased intracranial pressure, and unstable hemodynamics) for HFNO, anesthesiologists should consider risk factors when determining the duration of tubeless anesthesia maintenance. In previous studies, elevated body mass index (BMI) was the only significant predictor of oxygen desaturation during HFNO [4,13,30,37,40]. BMI > 30 kg/m2 was associated with a greater risk of requiring rescue ventilation, similar to alterations in respiratory physiology in obesity. Fat deposition primarily reduces pulmonary compliance by decreasing chest wall compliance [41]. In addition, airway resistance and airway pressure increase proportionally with the degree of obesity, which makes the propensity to overcome atelectasis and subsequent desaturation in obesity less likely [41,42]. These results demonstrate that tubeless anesthesia is safe and feasible for upper airway surgery in nonobese patients.
Surgical factors to consider
The effectiveness of HFNO depends on whether the patient has a patent upper airway. Therefore, patient disease characteristics are important factors affecting the applicability of tubeless anesthesia. Tubeless anesthesia is very helpful for posterior lesions because the endotracheal tube may hinder the surgical field [10,33]. Tubeless anesthesia can be a good option for supraglottic and glottic tumors because endotracheal intubation is challenging. Despite providing tubeless anesthesia and partial obstruction from surgical instruments, especially when the lesion is located in the glottis, transcutaneous CO2 levels are increasing more rapidly [7].
Although several advantages associated with surgery have been advocated, fire safety should be considered in LMS using lasers. Ignition can occur in the presence of an oxygen-rich environment, an oxidizer (tissue or plastic), or an energy source (CO2-laser). Therefore, airway surgeries using lasers under HFNO have the potential risk of airway fires. However, successful case reports of orolaryngeal surgeries using laser and electrocautery with HFNO have been published [30,33,35,40]. They revealed that there was no risk of airway fire despite delivering 100% oxygen at up to 70 L/min because there were no combustible materials such as polyvinyl chloride endotracheal tubes or gauze. It has been concluded that the risk of flammability does not increase with HFNO in laryngeal surgery using a laser unless there is a flammable foreign object. In contrast, animal simulation studies using electrocautery and lasers have revealed airway fires in HFNO [43,44]. According to these reports, removing combustible materials from the surgical field could greatly increase safety, but a high FiO2 of over 80% with interrupted laser strikes is highly likely to cause a sustained flame because human tissue and smoke can still serve as fuel and ignite under HFNO. Because there are limited clinical reports supporting the use of HFNO with pure oxygen and laser, the use of low FiO2 before the use of laser or electrocautery is recommended to minimize energy application and achieve safe airway surgery with HFNO.
A strategy for tubeless anesthesia
A secure protocol for tubeless anesthesia during upper airway surgery should be established and implemented. Before general anesthesia is induced, preparation for rescue endotracheal intubation or jet ventilation should be confirmed. For preoxygenation, the patients should be positioned supine with their heads elevated at 10–20°. HFNO with an oxygen concentration of 100% at 30–40 L/min is recommended for at least 3 min. Pre-oxygenation can be performed with the mouth open or closed. After preoxygenation, intravenous induction should be performed, usually with propofol and remifentanil in a target-controlled infusion, which should be titrated to the patient’s hemodynamics and a bispectral index (BIS) target of 40–60. Upper airway patency should be maintained with jaw thrust after loss of consciousness until surgical laryngoscopy is performed. Rocuronium (0.6 mg/kg) can be administered as a repeat bolus according to the TOF during surgery. Tubeless anesthesia without neuromuscular blockade has been explored; however, it has not been generally accepted because of the surgical difficulties caused by involuntary movement. Immediately after inducing general anesthesia, HFNO should be maintained at 100% oxygen at a flow rate of 70 L/min during non-laser upper airway surgery. At the end of the surgery, a supraglottic airway or endotracheal tube can be inserted for ventilation during recovery from general anesthesia.
Instruments for monitoring tubeless anesthesia
In addition to routine monitoring of blood pressure, electrocardiography, oxygen saturation, and BIS for general anesthesia, there are some helpful and noninvasive instruments for monitoring patient safety during tubeless anesthesia.
Arterial blood gas analysis is the gold standard for measuring the partial pressure of arterial CO2. However, this method is invasive. End-tidal CO2 (EtCO2) concentration monitoring is a noninvasive method for predicting PaCO2 which is routinely used in intubated patients, but is not possible in tubeless anesthesia. Transcutaneous partial pressure of CO2 (TcCO2) monitoring is a new noninvasive method that can continuously and reliably measure PaO2 in a patient without endotracheal intubation during surgery [45-48]. Even TcCO2 is found to reflect PaCO2 more accurately than EtCO2. Therefore, TcCO2 monitoring is mandatory during tubeless anesthesia to predict the respiratory status of patients, as hypercarbia is the most critical factor that anesthesiologists should manage with caution.
Another useful instrument for tubeless anesthesia is oxygen reserve index (ORI™, Masimo Co., USA). ORI is a new parameter that noninvasively indicates real-time oxygenation reserve status [49]. In range of PaO2 100–200 mmHg, ORI varies between 0.00 and 1.0, which reflects the patient’s mild hypoxemic status. Considering the ventilatory status, tubeless anesthesia should be accompanied by monitoring of these specific instruments to prevent complications associated with apnea.
CONCLUSION
Tubeless anesthesia via HFNO is not new, but is an early adoption for anesthesiologists in upper airway surgery. If anesthesiologists and surgeons select appropriate patients and apply tubeless anesthesia based on sufficient experience, it could be a good alternative to conventional anesthesia. To establish a safe surgical environment with tubeless anesthesia, meticulous patient monitoring along with appropriate backup plans, including endotracheal intubation and HFJV, should be supported.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Se-Hee Min, Jeong Hwa Seo. Formal analysis: Se-Hee Min. Writing - original draft: Se-Hee Min.Writing - review & editing: Se-Hee Min, Jeong Hwa Seo. Supervision: Se-Hee Min, Jeong Hwa Seo.