Perioperative management of patients receiving non-vitamin K antagonist oral anticoagulants: up-to-date recommendations
Article information
Abstract
Indications of non-vitamin K antagonist oral anticoagulants (NOACs), consisting of two types: direct thrombin inhibitor (dabigatran) and direct factor Xa inhibitor (rivaroxaban, apixaban, and edoxaban), have expanded over the last few years. Accordingly, increasing number of patients presenting for surgery are being exposed to NOACs, despite the fact that NOACs are inevitably related to increased perioperative bleeding risk. This review article contains recent clinical evidence-based up-to-date recommendations to help set up a multidisciplinary management strategy to provide a safe perioperative milieu for patients receiving NOACs. In brief, despite the paucity of related clinical evidence, several key recommendations can be drawn based on the emerging clinical evidence, expert consensus, and predictable pharmacological properties of NOACs. In elective surgeries, it seems safe to perform high-bleeding risk surgeries 2 days after cessation of NOAC, regardless of the type of NOAC. Neuraxial anesthesia should be performed 3 days after cessation of NOACs. In both instances, dabigatran needs to be discontinued for an additional 1 or 2 days, depending on the decrease in renal function. NOACs do not require a preoperative heparin bridge therapy. Emergent or urgent surgeries should preferably be delayed for at least 12 h from the last NOAC intake (better if > 24 h). If surgery cannot be delayed, consider using specific reversal agents, which are idarucizumab for dabigatran and andexanet alfa for rivaroxaban, apixaban, and edoxaban. If these specific reversal agents are not available, consider using prothrombin complex concentrates.
INTRODUCTION
Atrial fibrillation, the most frequently encountered arrhythmia, is associated with thromboembolism and stroke which need to be prevented amongst other therapies involving rhythm control [1]. For that purpose, vitamin K antagonist, warfarin, has long been used despite its inconstant and unpredictable anticoagulation effect which requires constant dose adjustments and laboratory monitoring [2,3]. Non-vitamin K antagonist oral anticoagulants (NOACs), also called direct oral anticoagulants (DOACs), were developed as an alternative to warfarin in order to overcome the aforementioned pharmacological limitations of warfarin [4,5].
Based on cumulating clinical evidence stemming from large multicenter randomized trials, NOACs were shown to be non-inferior to warfarin in preventing stroke and thromboembolism with lower risk of serious bleeding events in patients with non-valvular atrial fibrillation [6–9]. Additionally, owing to the reliable pharmacokinetic properties of NOACs, they were prescribed in fixed doses without laboratory monitoring. This led to the incorporation of NOACs as valuable therapeutic options for anticoagulation in atrial fibrillation patients, by the American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) in 2014 [1]. With the emergence of newer evidences showing favorable clinical efficacy and safety of NOACs in various subsets of patients [10–12], focused update of the 2014 guideline by the AHA/ACC/HRS in 2019 recommended the use of NOACs as first-line agents over warfarin in eligible patients with non-valvular atrial fibrillation (i.e., except those with moderate-to-severe mitral stenosis or a mechanical heart valve) [13]. A similar preference of NOACs over warfarin was also advocated by the European Heart Rhythm Association in 2018 [14]. Furthermore, current indications of NOACs include treatment or prevention of deep vein thrombosis and pulmonary embolism, promoting its widespread use [15–17].
Accordingly, increasing number of patients presenting for surgery are exposed to NOACs, despite the fact that NOACs can inevitably increase risk of bleeding as other anticoagulants. This review aimed to provide essential knowledge on NOACs, and evidence-based up-to-date recommendations regarding the perioperative management of NOACs.
PHARMACOLOGICAL ASPECTS OF NOACS
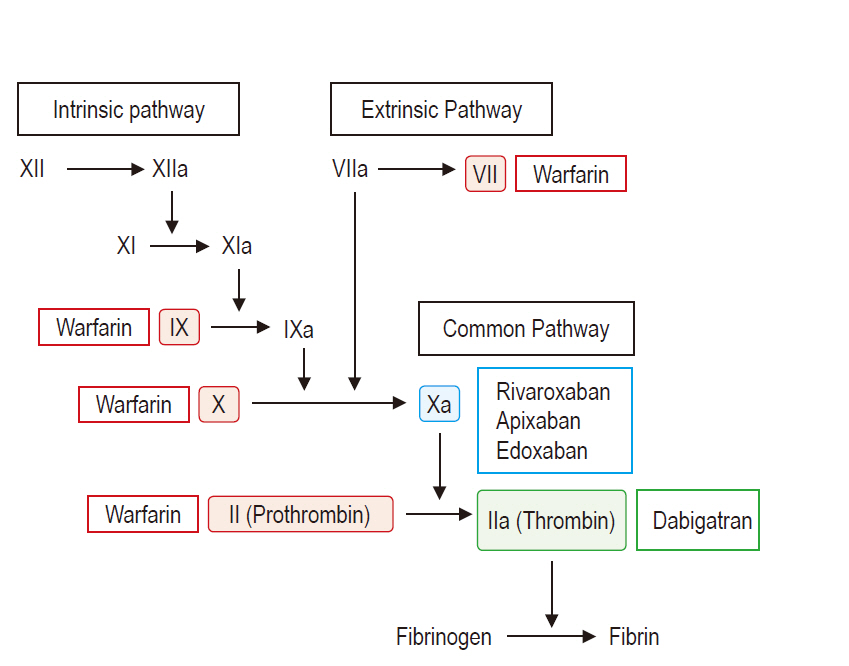
Unlike warfarin which affects multiple vitamin K-dependent coagulation factors II, VII, IX, and X, NOACs were designed to directly act on a single target factor to yield a more predictable anticoagulant response [18]. Currently, there are 4 approved NOACs which can be divided in 2 types depending on their action mechanisms (Fig. 1): the direct thrombin inhibitor (dabigatran) [19], and the direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban) which imped the conversion of prothrombin to thrombin [20].
Compared to warfarin, the pharmacokinetic advantages of NOACs include a more rapid onset (time to peak: 1 to 3 h), shorter elimination half-life (5 to 15 h), lower predisposition to food and drug interaction (do not require restriction on vitamin K-containing food), and a more predictable anticoagulation effect (Table 1) [18,20]. These features allow fixed-dose administration in the absence of routine therapeutic laboratory monitoring. Thus, the major studies that compared the efficacy of NOACs with warfarin did not carry out dose adjustments or perform routine laboratory testing to detect the therapeutic level of NOACs [6–9].
NOACs undergo hepatic metabolism and plasma hydrolysis, and are substrates for the multidrug transporter P-glycoprotein and CYP 3A4 metabolism, while edoxaban exists mostly in an unchanged form in plasma, being minimally metabolized through CYP 3A4 [18,20]. Therefore, concomitant administration of drugs that strongly inhibit these pathways, such as dronedarone, amiodarone, and verapamil, may increase the active drug levels of the NOACs, except edoxaban [21]. NOACs are mostly excreted via the kidney, and approximately 80%, 33%, 27%, and 50% of dabigatran, rivaroxaban, apixaban, and edoxaban, respectively, undergo unchanged renal elimination, mandating the need for regular monitoring of renal function [4].
BLEEDING RISK ASSOCIATED WITH NOACS AND REVERSAL AGENTS
Although NOACs were shown to be associated with lower rates of intracranial and life-threatening bleeding when compared with warfarin [22], all anticoagulants have the innate potential to increase bleeding risk. In patients with non-valvular atrial fibrillation treated with NOACs, the estimated pooled incidence of hemorrhagic stroke was 0.4% [22]. In contrast, NOACs conferred a 1.5-fold increased risk of gastrointestinal bleeding, which accounted for approximately 90% of the major extracranial bleeding, compared to warfarin [6,7,9,23], with an overall 3.3% incidence of major bleeding [24].
Unlike warfarin which can be readily reversed by vitamin K, prothrombin complex concentrates (PCC), or fresh frozen plasma (FFP), there were no available reversal agents for NOACs during the major phase III clinical trials. Still, the fatality rate of patients on NOACs who exhibited major bleeding was similar or even less than that of patients on warfarin [22]. Nonetheless, bleeding complications happen, whether spontaneous in nature or associated with an invasive procedure/surgery. Accordingly, the reversal agents developed for NOACs were shown to be effective in stopping major bleeding events [25–27]. Although there is limited clinical evidence on these agents due to the unexpected nature of spontaneous bleeding events, two reversal agents were approved by the U.S. Food and Drug Administration (FDA): idarucizumab for dabigatran reversal and andexanet alfa for rivaroxaban and apixaban reversal [13]. Additionally, another reversal agent, ciraparantag, which can theoretically reverse the anticoagulation effects of all NOACs is being studied, and the results are being awaited [26].
Idarucizumab
Idarucizumab is a humanized monoclonal antibody fragment (antigen-binding fragment; Fab) which has a 350-fold higher binding affinity to dabigatran than thrombin [28]. Thus, it frees thrombin from dabigatran inhibition and immediately reverses the anticoagulation effect in a dose-dependent manner after intravenous administration [29]. The recommended administration protocol suggests two 2.5 g intravenous boluses (total of 5 g), each given in 50 ml infusion over 5–10 min in order to reverse 99% of the estimated dabigatran’s anticoagulation effect [27]. Although its elimination half-life is approximately 45 min, doses of 2 g or more have been shown to exert a complete and sustained effect over 72 h [29]. Yet, administration of a second dose of 5 g may be considered, if necessary.
While relevant clinical evidence is limited, overall, idarucizumab has been shown to be effective in reversing dabigatran-induced major bleeding. Its efficacy has also been shown in patients requiring emergency surgery, and normal hemostasis with its use could be confirmed by the surgeons in approximately 93% of the patients, while the incidence of thromboembolic events at 30 days after idarucizumab administration was 4.8% [27]. Thus, despite the paucity of related clinical evidence, the U.S. FDA has approved the use of idarucizumab for patients receiving dabigatran who exhibit life-threatening bleeding or require emergent surgery as incorporated in the 2019 update of AHA/ACC/HRS guidelines (class I recommendation, level of evidence B-NR) [13].
Andexanet alfa
Andexanet is an inactive variant of human recombinant factor Xa in which the active serine-residue is replaced by alanine to eliminate its catalytic activity and to prevent the formation of prothrombin complex [30]. Thus, theoretically, andexanet can reverse the anticoagulant effect of all NOACs that are factor Xa inhibitors, except dabigatran. Andexanet’s binding affinity to factor Xa inhibitors is similar to that of the native factor Xa [26].
Considering the importance of a specific reversal agent, the U.S. FDA has recently approved (accelerated-approval pathway) the use of andexanet alfa for reversal of rivaroxaban- or apixaban-induced life-threatening or uncontrolled bleeding, based on the limited evidence from healthy volunteers, and this has newly been incorporated in the 2019 update of AHA/ACC/HRS guidelines (class IIa recommendation, level of evidence B-NR) [13]. Shortly after the approval of andexanet and the publication of relevant focused update by the AHA/ACC/HRS in 2019, full study results of a prospective multicenter trial addressing the efficacy of andexanet alfa for bleeding associated with factor Xa inhibitors (ANNEXA-4 trial) were published [25]. In that study, treatment with andexanet resulted in immediate reduction of anti-factor Xa activity (92% reduction in both apixaban and rivaroxaban), yielding good hemostatic efficacy in 82% of the patients at 12 h, with a thromboembolic event rate of 10% at 30 days.
Current dosing recommendations are intravenous bolus over 15–30 min, followed by 2 h of continuous infusion: 1) 400 mg bolus, 480 mg infusion in patients who received rivaroxaban (last intake > 7 h) or apixaban, and 2) 800 mg bolus, 960 mg infusion in patients who received rivaroxaban within 7 h (or unknown timing) or edoxaban [14,25].
Notably, andexanet also binds to heparin-antithrombin III complex, reversing the actions of low molecular-weight heparin and unfractionated heparin [31].
Ciraparantag
Ciraparantag is a synthetic cationic molecule that was developed to reverse the anticoagulation effect of unfractionated or low molecular-weight heparin via non-covalent hydrogen linkage and charge-charge interaction [32]. Also, it directly binds to Xa inhibitors and thrombin inhibitors in a similar manner [20]. Thus, it would be able to reverse the anticoagulation effect of all NOACs, irrespective of their action mechanism. Available data which show its promising results in reversing the anticoagulation effect of all NOACs are limited to animal studies or healthy volunteers [33]. Currently, ciraparantag is not approved for clinical use.
ELECTIVE SURGERY AND NOACS
Approximately 10% of patients who require oral anticoagulants undergo surgery or invasive procedures yearly [34,35]. For patients’ safety, it is unarguable that NOACs should be appropriately discontinued in patients undergoing intermediate/high bleeding risk procedures. So far, clinical evidence is not enough to support a uniform guideline, and current recommendations by responsible societies including the AHA, European Heart Rhythm Association, and the European Society of Anaesthesiologists published in 2017, 2018, and 2017, respectively, are largely based on limited clinical studies and expert consensus [14,20,36–38]. Nonetheless, NOACs’ reliable pharmacologic profiles would permit safe surgery and recovery by maintaining the balance between bleeding and thromboembolic risk.
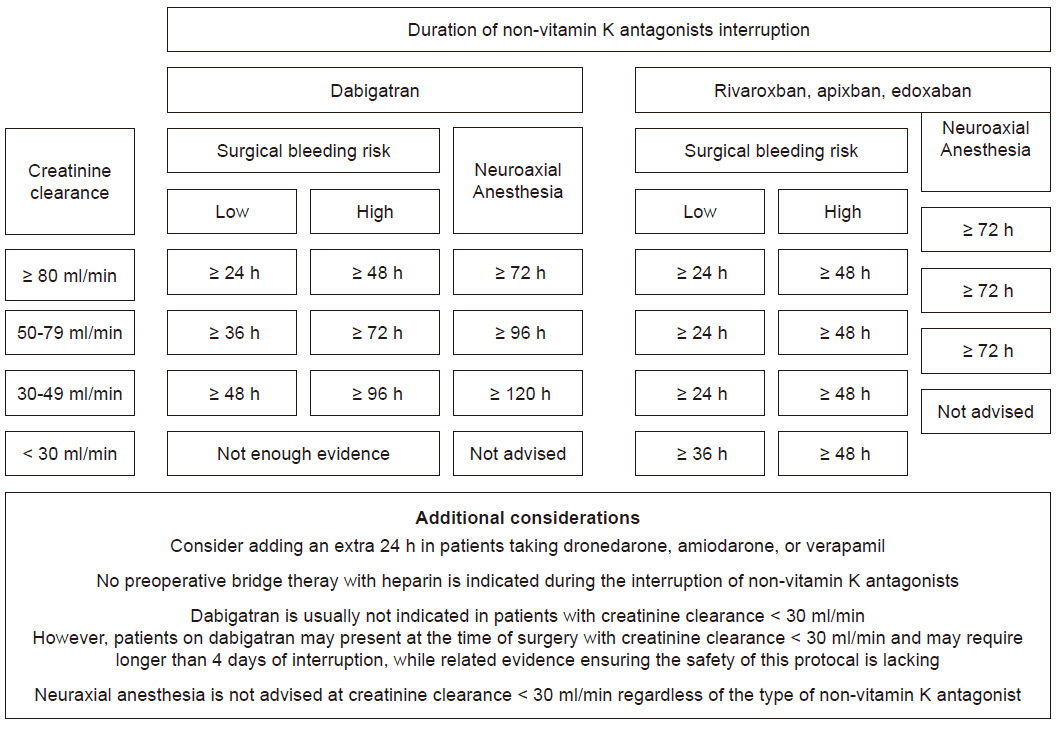
To provide the patients with a safe perioperative milieu, two major questions arise: 1) when to discontinue NOACs before surgery, and 2) the need for bridge-anticoagulation therapy. First, NOACs have a relatively short half-life, ranging from 5 to 15 h in patients with normal renal function [20]. Thus, discontinuing NOACs for 2 days before surgery with high bleeding risk would allow negligible residual drug concentration (usually < 10% corresponding to discontinuation for 3 to 4 half-lives), whereas discontinuation for 1 day would suffice for surgeries or procedures with low bleeding risk (15 to 25% residual activity) [38]. Notably, the elimination of NOACs depends on the renal function to various degrees which must be assessed and properly taken into consideration before surgery. Based on creatinine clearance (CrCl), dabigatran needs to be discontinued for 3 days and 4 days with CrCl of 50 to 79 ml/min and 30 to 49 ml/min, respectively [14]. In case of rivaroxaban, apixaban, and edoxaban, 2 days would suffice in most of the patients, regardless of the renal function. In all patients, further consideration should be given when receiving concomitant dronedarone, amiodarone, or verapamil, such as discontinuation for an additional 1 day when the thromboembolic risk is not high [14,21].
Second, preoperative bridge therapy with heparin is usually recommended for patients at high-risk of thromboembolic complication, such as those with mechanical heart valve [13]. However, as NOACs are currently not indicated in patients with mechanical heart valve, this recommendation does not apply to patients receiving NOACs. Also, the short elimination half-lives of NOACs require a short duration of cessation before surgery as opposed to the 5 days required in warfarin [20,39]. Moreover, discontinuation of NOACs has not been shown to result in rebound hypercoagulability [7–9]. Indeed, sub-analysis of major NOAC trials showed a low incidence of thromboembolic events ranging from 0.2 to 0.6% without bridging, whereas bridging with heparin resulted in increased bleeding complications without any benefit in terms of thromboembolic risk [24,40,41]. Thus, bridging therapy for NOACs in the preoperative period is currently not recommended, but it should be restarted after surgery as soon as possible [14].
So far, clinical evidence adhering to the above-mentioned recommendations for interruption of NOACs before surgery resulted in a similar rate of postoperative bleeding events when compared to patients receiving warfarin [38]. Data from pivotal NOACs studies including the German and Canadian registry, reported major bleeding incidences ranging from 0.6 to 3% after surgery [24,42]. Recently, full data from the perioperative anticoagulation use for surgery evaluation (PAUSE) cohort trial was published, and so far, it is the largest prospective multicenter trial that provided more insights regarding the perioperative NOACs management [43]. In that study, NOACs were discontinued for 1 day and 2 days for low- and high-bleeding risk procedures, respectively. In patients receiving dabigatran, longer interruption was applied accounting for CrCl. NOACs were resumed 1 day and 2 to 3 days after low- and high-bleeding risk surgeries, respectively. Overall, major bleeding rates were less than 2%, and the rates of thromboembolism were less than 1%, showing similar efficacies as with warfarin and confirming the clinical usefulness of the simple management strategy.
Neuraxial anesthesia, such as spinal or epidural, is considered a high-bleeding risk procedure. The most recent recommendations by the American Society of Regional Anesthesia and Pain Medicine published in 2018 approaches NOACs on a more conservative basis considering the even more limited clinical evidence in that regard [44]. Dabigatran was recommended to be discontinued for 3, 4, and 5 days in patients with CrCl of > 80, 50 to 79, and < 50 ml/min, respectively. Rivaroxaban, apixaban, and edoxaban were recommended to be discontinued for 3 days before Neuraxial anesthesia.
A summary of the current recommendations incorporating the most recent clinical evidences are displayed in Fig. 2.
EMERGENT/URGENT SURGERY AND NOACS
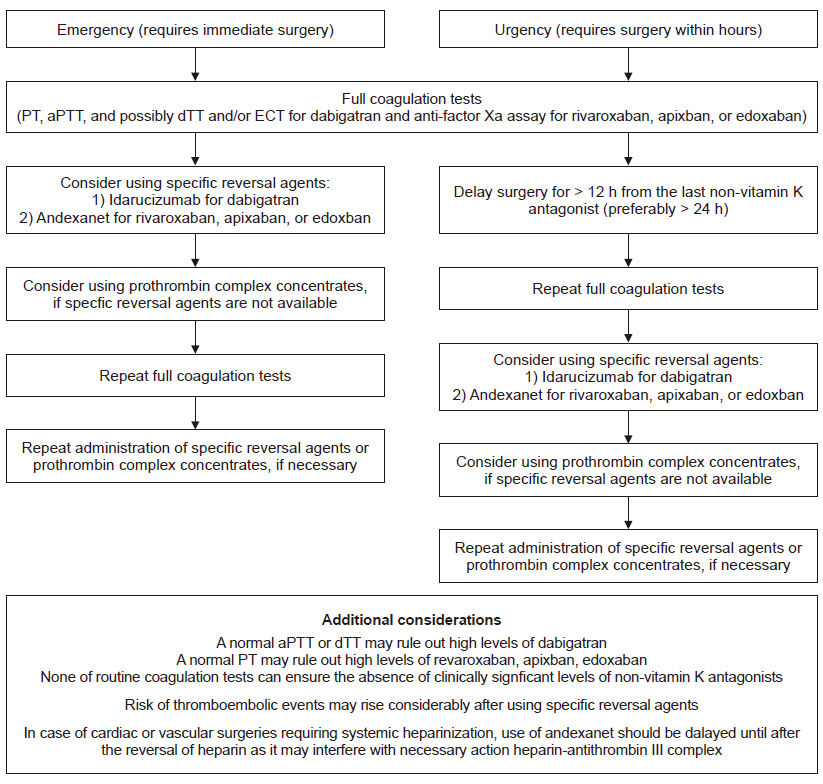
In an emergent situation, NOACs should be immediately stopped, and the following detailed knowledge should be acquired: 1) type of NOAC used, 2) last time of intake, 3) renal function, and 4) full panel of coagulation tests (prothrombin time [PT], activated partial thromboplastin time [aPTT], and possibly chromogenic anti-factor Xa assay, or diluted thrombin time [dTT]/ecarin-based assays [ECA]) [14].
In life-threatening or salvage emergencies such as cardiac, vascular, or neurosurgical surgeries that cannot be delayed even for a few hours, consideration should be given to administer specific reversal agents: idarucizumab for dabigatran and andexanet for rivaroxaban, apixaban, and edoxaban [14]. Yet, in case of surgeries requiring systemic heparinization, such as cardiac or vascular, the use of andexanet may be deferred until heparin reversal with protamine, as it may inhibit the anticoagulant effect of heparin [31] which is an absolute necessity for surgery. It should be noted that the incidence of thromboembolic events showed a dramatic increase to 18% after administration of the reversal agents [45,46], whereas it was less than 1% in case of planned interruption of NOACs [43]. Thus, apart from their high cost, the use of specific reversal agents should be carefully decided.
If these specific reversal agents are not accessible, PCC may be given, although the supporting clinical evidence is limited and controversial [47–49]. Suggested regimens of PCC include 2 doses of 4-factor PCC or an initial bolus of 50 IU/kg followed by an additional 25 IU/kg if necessary [14]. FFP is not likely to effectively reverse NOACs, unless used in large volumes (at least 8–16 units of FFP would equal the dose of 25–50 IU/kg of 4-factor PCC), and thus, it is not recommended for that purpose [50]. Also, without related clinical evidence, other therapies aimed at reducing perioperative blood loss, such as tranexamic acid, which is an antifibrinolytic agent that may be considered due to its proven efficacy and relative safety in major surgeries [36].
In urgent cases that need to be done within hours, consideration should be given to delaying the surgery for at least 12 h (preferably 24 h) after the last NOAC administration, as a considerable amount of the given NOAC would be eliminated within this timespan. After delay, the coagulation tests should be performed again. Routine coagulation tests, such as PT and aPTT, cannot quantify or determine the activity of any given NOAC. Yet, a normal dTT or aPTT would most likely exclude high therapeutic levels of dabigatran, whereas normal PT would rule out high levels of rivaroxaban as well as edoxaban (to a lesser extent) [51]. Despite these associations, it should be noted that none of the routine coagulation tests ensure the absence of clinically significant levels of NOACs even when the test results are normal [51]. Preferably, specific tests to measure the activity of NOACs should be performed to guide the need for reversal agents. These include ECA for dabigatran and anti-factor Xa assays for rivaroxaban, apixaban, or edoxaban [52–54]. However, these tests may not be readily available in all institutions, and clinical evidence on targeting therapies according to the specific test results is lacking, leaving the clinical judgment at the discretion of the attending physician.
In case of dabigatran, hemodialysis may be considered, as it has been shown that approximately 50 to 60% of the drug was removed after 4 h of hemodialysis administration [55]. But, the practicability of hemodialysis remains questionable considering that it requires anticoagulation. Other NOACs are unlikely to be removed by hemodialysis due to their high-protein binding properties [56].
Other non-specific measures to decrease its absorption is the use of activated charcoal (30 to 50 g), which has been shown to effectively reduce the absorption of recently overdosed NOACs [36]. Thus, it may be considered in patients who ingested NOAC within 2 to 4 h before urgent surgery. However, its efficacy in patients who received a prescribed dose of NOAC, and not accidental overdosed, remains questionable considering the side effects of charcoal including nausea/vomiting and aspiration [57].
A summary of the current recommendations incorporating the most recent clinical evidences are displayed in Table 2 and Fig. 3.

Reversal Agents and Alternative Options for Patients on Non-vitamin K Antagonist Requiring Emergent/Urgent Surgery
CONCLUSIONS
Emerging evidence advocates the use of NOACs over warfarin in patients with non-valvular atrial fibrillation, with indications expanding to patients at increased risk of deep vein thrombosis or pulmonary embolism [13,14,16,58]. As the field of anesthesiology has expanded to perioperative medicine, critical care, and pain medicine, patients receiving NOACs will be encountered more frequently in our daily practice. Practice guidelines regarding the management of NOACs should be available in every institution incorporating the recent evidence regarding the interruption strategy and specific reversal agents to provide optimal care in patients requiring surgeries.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: Kwang-Sub Kim, Jae-Kwang Shim. Data acquisition: Sarah Soh, Jong Wook Song. Supervision: Young-Lan Kwak. Writing—original draf: Kwang-Sub Kim, Jae-Kwang Shim.