Comparison of postoperative pulmonary complications between sugammadex and neostigmine in lung cancer patients undergoing video-assisted thoracoscopic lobectomy: a prospective double-blinded randomized trial
Article information
Abstract
Background
Reversal of neuromuscular blockade (NMB) at the end of surgery is important for reducing postoperative residual NMB; this is associated with an increased risk of postoperative pulmonary complications (PPCs). Moreover, PPCs are associated with poor prognosis after video-assisted thoracoscopic surgery (VATS) for lobectomy. We compared the effects of two reversal agents, sugammadex and neostigmine, on the incidence of PPCs and duration of hospital stay in patients undergoing VATS lobectomy.
Methods
After VATS lobectomy was completed under neuromuscular monitoring, the sugammadex group (n = 46) received sugammadex 2 mg/kg, while the neostigmine group (n = 47) received neostigmine 0.05 mg/kg with atropine 0.02 mg/kg after at least the third twitch in response to the train of four stimulation. The primary outcome was incidence of PPCs. The secondary outcomes were duration of hospital stay and intensive care unit (ICU) admission.
Results
There was no significant difference in the incidence of PPCs for both the sugammadex and neostigmine groups (32.6% and 40.4%, respectively; risk difference = 0.08; 95% confidence interval = [−0.12, 0.27]; P = 0.434). The lengths of hospital (P = 0.431) and ICU (P = 0.964) stays were not significantly different between the two groups.
Conclusions
The clinical use of sugammadex and neostigmine in NMB reversal for patients undergoing VATS lobectomy was not significantly different in the incidence of PPCs and duration of hospital and ICU stay.
INTRODUCTION
The primary goal of enhanced recovery after surgery (ERAS) in lung surgery is to optimize perioperative and intraoperative management to reduce postoperative complications and decrease the length of hospital stay [1,2]. Particularly, in the field of anesthesia management, neuromuscular blockade (NMB) and its reversal are important because deep NMB is necessary during thoracoscopic lung surgery to secure a good field of view and operating space for the surgeon. Additionally, the choice of reversal agent used to reverse NMB after surgery is important for proper recovery by reducing postoperative residual NMB (PRNMB) [1,3]; this is important for patient safety in the early postoperative period because PRNMB increases the incidence of adverse respiratory events, such as impaired upper airway function, increased risk of aspiration, and respiratory insufficiency [4–6].
As a cholinesterase inhibitor, neostigmine mainly inhibits acetylcholine breakdown, increases acetylcholine levels in the neuromuscular junction, and enhances the ability of acetylcholine to compete with non-depolarized muscle relaxants at the receptor [3,7]. However, in several studies on the routine use of neostigmine, the incidence of PRNMB on arrival to the post-anesthesia care unit did not change. Furthermore, because it is not a direct neuromuscular blocking agent antagonist, the reversal of NMB using neostigmine is associated with an increased incidence of postoperative atelectasis, desaturation, postoperative pulmonary complications (PPCs), and longer hospital stay [7,8]. In addition, PRNMB is associated with an increased risk of PPCs [7–10].
In contrast, de Menezes et al. [11] reported that under neuromuscular monitoring, sugammadex led to a complete reversal of NMB after incomplete reversal by neostigmine. Similarly, sugammadex is a selective relaxant binding agent that rapidly reverses moderate or deep NMB. It is a synthetically modified gamma-cyclodextrin with a hydrophilic exterior and a hydrophobic core, specifically designed to encapsulate steroidal neuromuscular blocking agents [3,6,7]. Sugammadex reverses NMB more rapidly and effectively than cholinesterase inhibitors, even at deep block levels, by directly binding to steroidal neuromuscular blocking agents without residual muscle relaxation [3,8].
Therefore, in the reversal of NMB at the end of surgery, it is thought that sugammadex would contribute to reducing PPCs by reducing PRNMB. However, few studies have assessed the reversal of NMB in video-assisted thoracoscopic (VATS) lobectomy. Thus, we hypothesized that sugammadex would reduce PPCs and the length of hospital stay in patients undergoing VATS lobectomy, compared with the traditionally and widely used neostigmine.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board (no. DAUHIRB-18-047) and was conducted between April 2018 and May 2020. Informed written consent was obtained from patients who were scheduled for elective surgery before enrollment and who voluntarily agreed to participate. One hundred and two patients were enrolled, who underwent VATS lobectomy, had an American Society of Anesthesiologists physical status of I–III, and were over 18 years old. Cases with open conversion during the operation were excluded. This randomized and double-blinded prospective study was registered at the Korea Clinical Research Information Service (no. KCT 0003891).
Using a computer-generated random number, 102 participants were randomly allocated to the neostigmine group (n = 51) or sugammadex group (n = 51) in parallel; this was performed by the main researcher (1:1 assignment ratio), who did not participate in anesthesia and was not involved in data collection. Ten milliliters of study drug solution was prepared in normal saline by the main researcher, based on the patient’s body weight. The sugammadex group was administered sugammadex 2 mg/kg, while the neostigmine group was administered neostigmine 0.05 mg/kg (maximum 5 mg) with atropine 0.02 mg/kg. The study drug was labeled as reverse after being shielded with a silver foil, and it was delivered to the anesthesia nurse.
All patients were administered 0.2 mg glycopyrrolate intramuscularly and 20 mg famotidine intravenously as premedication. Non-invasive blood pressure, electrocardiography, pulse oximetry, bispectral index (BIS) (Aspect Medical Systems, USA), and neuromuscular monitoring (TOFscan®, Dräger, France) were performed when the patients arrived at the operating room.
Anesthesia was induced by two blinded anesthesiologists, each of whom had experience with over 50 cases of one-lung ventilation, using propofol 2 mg/kg and rocuronium 0.6 mg/kg for NMB. We performed bag-mask ventilation with 100% oxygen. After confirming muscle relaxation (no reaction in response to the train of four [TOF] stimulation), we performed endotracheal intubation using a double lumen endobronchial tube (DLT) (ShileyTM, Covidien, USA). An arterial catheter and a central venous catheter were inserted. Anesthesia was maintained with sevoflurane 1.5–2.5 vol% to maintain a BIS of 40–60; remifentanil infusions (0.05–2 ㎍ /kg/min) were administered for pain control. To maintain a deep block, vecuronium (0.01 mg/kg) was administered as required (first twitch of the TOF response) [12]. Oxygen concentration in an air/oxygen mixture was adjusted to 100% according to arterial blood gas analysis (ABGA) results. During severe hypoxemia, continuous positive airway pressure was applied to the non-dependent lung [13]. The end-tidal CO2 concentration was maintained between 35 and 40 cmH2O, and the following lung-protective ventilation strategies were implemented [2,13]: low tidal volume (4–6 ml/kg), positive end-expiratory pressure, and lung recruitment. Flexible fiber-optic bronchoscopy (ED 3.1 mm, Olympus Optical, Japan) was used at least twice to confirm correct placement of the DLT immediately following intubation and repositioning. The intra-cuff pressure was maintained between 10 and 20 cmH2O using a cuff pressure manometer (Cuff Pressure Gauge, VBM Medizintechnik, Germany ).
When one-lung ventilation was complete, administration of vecuronium was terminated, and a patient-controlled analgesia device (GemStarTM Infusion System, Hospira, Inc., USA) was connected to the intravenous line. It contained fentanyl 30 µg/kg and ramosetron 0.6 mg in normal saline with a total volume of 100 ml, and it was delivered at 1 ml/h as a background infusion with a 1 ml bolus dose (10 min lock-out time). To reduce opioid consumption, 0.5% ropivacaine was infused at 5 ml/h through a pain buster (Pain Relief System, Halyard Health, Inc., USA) intercostally before the end of surgery [2].
At the end of surgery, the labeled NMB reversing agent was administered at the reappearance of the third twitch in response to the TOF stimulation. Tracheal and oral secretions were gently suctioned once. After suction was applied, lung recruitment was performed. Neuromuscular monitoring continued until the end of anesthesia, and the DLT was removed when the recovering TOF ratios (TOFR) reached 0.9, which is considered adequate recovery from NMB [7].
Postoperative care included chest tube use, intensive care unit (ICU) stay, intubation, and ventilation; postoperative hospital stay was routinely implemented according to VATS lobectomy guidelines at the department of thoracic surgery. Intraoperative ABGA, operation time, and duration of anesthesia were recorded.
The primary outcome was the incidence of PPCs including prolonged air leak, pneumonia and atelectasis, desaturation, and reintubation as confirmed from progress and discharge records. Prolonged air leakage was defined as an air leak present on day 6 after surgery. Pneumonia and atelectasis were diagnosed based on radiologic findings of the postoperative chest radiograph. Desaturation was defined as saturation levels less than 95% after removing the oxygen mask. Reintubation was performed when respiratory failure occurred. Atrial fibrillation was diagnosed based on electrocardiography findings, when the patient complained of symptoms, such as dyspnea, palpitation, and fatigue. Pulmonary thromboembolism was diagnosed based on computed tomography and D-dimer test results. The secondary outcomes were the length of hospital stay and duration of ICU stay.
Statistical analyses
Based on the results of Cho et al. [3], in which the overall incidence of PPCs was 54.8% in the control group and 26.3% in the experimental group, 46 subjects were needed in each group to detect statistically significant differences (α = 0.05, power = 0.80). Thus, 51 patients per group were enrolled to compensate for potential dropouts (drop rate = 10%).
Data were presented as means ± SD, number of patients (%) or medians (1Q, 3Q). Categorical variables were analyzed using the chi-squared or Fisher’s exact test. Continuous variables were analyzed using Student’s t-test. All reported P values were two sided, and P values less than 0.05 were considered statistically significant. Data were analyzed using SPSS version 26 software (IBM Corp., USA).
RESULTS
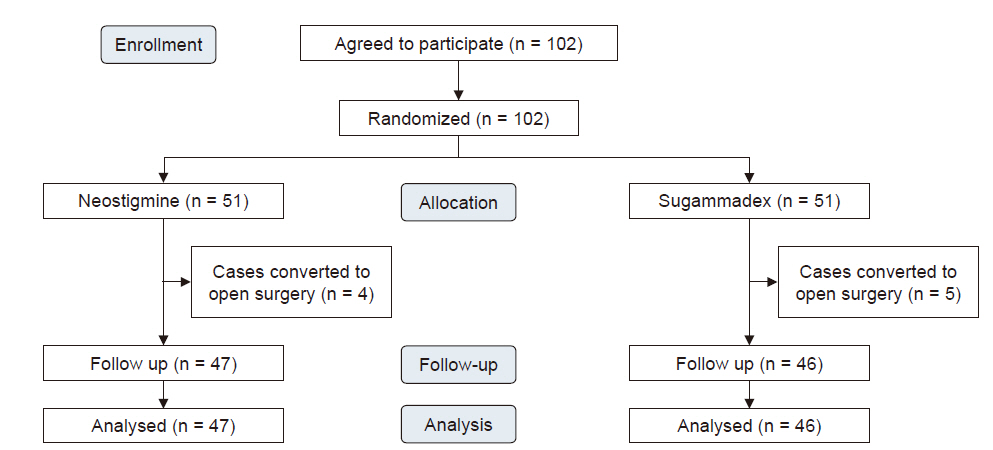
Of the 102 patients, nine were excluded due to conversion to open surgery (four in the neostigmine group and five in the sugammadex group) (Fig. 1). Table 1 shows the patient characteristics of both groups. There were no significant differences between the two groups in factors that could affect postoperative complications, such as smoking history, body mass index, American Society of Anesthesiologists physical status classification , lobectomy site, or medical history. Predictive postoperative forced expiratory volume in 1 s was statistically significant (P = 0.016); however, there is no clinical significance to this, since the absolute values were not low in either group.
In addition, there was no significant difference in anesthesia management during surgery in terms of the operation time, anesthetic time, ABGA results, and total vecuronium usage during surgery between the neostigmine and sugammadex groups (Table 2).
As shown in Table 3, the incidence of PPCs was not significantly different between the sugammadex and neostigmine groups (32.6% vs. 40.4%, respectively; risk difference = 0.08; 95% confidence interval = [−0.12, 0.27]; P = 0.434). There were no significant differences in terms of specific cardiopulmonary complications.
As shown in Table 4, there was no significant difference in the length of postoperative hospital stay (P = 0.431) or duration of ICU stay (P = 0.964) between the two groups.
DISCUSSION
Apart from PRNMB, anesthesiologists need to consider other factors affecting the incidence of postoperative complications. As a cholinesterase inhibitor, neostigmine is not a direct reversal, and it is associated with muscle weakness. Muscle weakness induced by neostigmine usually occurs due to administration of the drug at a higher dose after a nearly complete recovery of NMB. This is associated with respiratory impairment, including an increased risk of atelectasis, pulmonary edema, desaturation, and longer hospital stay [7,14]. Moreover, Abad-Gurumeta et al. [8] reported in a review article that even when extubation was achieved under high doses of neostigmine (> 0.6 mg/kg), at TOFR greater than 0.9, neostigmine administration was associated with atelectasis, pulmonary edema, tracheal re-intubation, and prolonged hospital stay. In addition, Schepens et al. [15] obtained computed tomography scans during the spontaneous breathing cycle (TOFR > 0.9) after administering neostigmine (0.06 mg/kg), sugammadex (15 mg/kg), or saline at a TOFR of 0.5 in rats. They found that rats being administered neostigmine exhibited a smaller relative contribution of diaphragm movement to the total change in lung volume compared with sugammadex or saline. This may be due to the effect of neostigmine on neuromuscular transmission because the residually occupied acetylcholine receptors may decrease the efficiency of neuromuscular coupling at the diaphragm. In our research, the dose of neostigmine was predetermined; a dose of 0.5 mg/kg above the light block (TOFR > 0.4) may cause muscle weakness in high-risk patients and may be associated with the occurrence of complications. Furthermore, the risk of cardiovascular complications is also relatively high with neostigmine because of hemodynamic changes caused by bradycardia and cardiac arrhythmias [7]. However, theoretically, sugammadex is a selective relaxant-binding agent and has a lower risk of cardiopulmonary complications than a combination of neostigmine and atropine [3,6,7,16]. Moreover, sugammadex possibly reduces the time required for complete recovery from NMB without increasing the incidence of hypersensitivity or anaphylactic reactions, even in deep blocks [16–18]. Furthermore, in a systematic review by Paton et al. [16], sugammadex was found to be more cost effective and potentially time-saving intraoperatively than neostigmine. Thus, we theorized that sugammadex was effective in reducing the incidence of PPCs compared with neostigmine, thereby helping to reduce the length of hospital stay.
Nevertheless, the primary outcome of this study showed no statistical significance regarding the effect of sugammadex and neostigmine in the reversal of moderate to light NMB after VATS lobectomy. In the present study, neuromuscular monitoring was performed until the end of anesthesia, as the drug dose was predetermined, owing to the double-blind method. Administration of the reversal agent was performed at the moderate to light block (over TOF count 3) and observed when TOFR reached 0.9, which is considered adequate recovery from NMB; this was followed by tracheal extubation because tracheal extubation at TOFR less than 0.9 was associated with more complications, including hypoxia, upper airway obstruction, oxygen desaturation, micro-aspiration, and reintubation, than extubation at TOFR greater than 0.9 [8]. This would have contributed to reducing the incidence of PRNMB after reversal in the neostigmine group, resulting in reduced incidence of PPCs. In addition, Togioka et al. [19] recently reported that among the 200 older adults undergoing prolonged surgery (high-risk subjects), there was no significant difference in the incidence of PPCs between the sugammadex and neostigmine groups (33% vs. 40%, respectively; odds ratio = 0.74; 95% confidence interval = [0.40, 1.37]; P = 0.30). They found that the contribution of reversal agent to decreased incidence of PPCs can be masked in the high-risk population through the impact of the disease on the incidence of complications. In the present study, the average age of the patient group was 64 years, and the average operation time was more than 3 h. In addition, the incidence of PPCs in patients undergoing VATS lobectomy is as high as 10–40% [20]. For these reasons, it is thought that the contribution of sugammadex to the reduction of PPCs may have been obscured by the high incidence of complications. Therefore, sugammadex did not show a statistical difference in reducing the incidence of PPCs compared with neostigmine (32.6% vs. 40.4%, respectively; risk difference = 0.08; 95% confidence interval = [−0.12, 0.27]; P = 0.434).
The secondary outcomes of this study showed no significant differences between the two groups in terms of the length of hospital stay, ICU admission, and chest tube removal. This suggests that there was no significant difference between the effect of neostigmine and sugammadex on the incidence of critical complications affecting the length of hospital stay and ICU stay. In addition, the department of thoracic surgery at our hospital routinely performs early chest tube removal to reduce pain and encourage early ambulation in order to reduce hospital stay, unless serious complications occur according to the ERAS, which may have also affected our results [1,7].
There were a few limitations in our study. First, it was not possible to confirm whether PRNMB was actually reduced because neuromuscular monitoring was not performed in the ICU after extubation. In addition, PRNMB and muscle weakness were not evaluated; therefore, it was not possible to confirm their correlation with PPCs.
In conclusion, in clinical use, the use of sugammadex in reversing NMB at a minimum TOF count of 3 did not reduce the incidence of PPCs in patients undergoing VATS lung lobectomy compared with neostigmine under quantitative neuromuscular monitoring. In addition, there was no difference in the duration of hospital and ICU stay between both groups. Therefore, more quantitative and large-scale studies in patient groups or surgeries with high incidence of complications are needed to demonstrate the benefits of sugammadex as a NMB reversal agent in the correlation between preoperative risk factors and PPCs.
Acknowledgements
This study was supported by Dong-A University.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: So Ron Choi. Data curation: Tae Young Lee, Seong Yeop Jeong, Joon Ho Jeong. Formal analysis: Seong Yeop Jeong. Methodology: So Ron Choi. Project administration: Joon Ho Jeong. Writing - original draft: Seong Yeop Jeong. Writing - review & editing: Tae Young Lee, Jeong Ho Kim. Investigation: Tae Young Lee. Resources: Jeong Ho Kim. Supervision: So Ron Choi.