|
 |
- Search
| Anesth Pain Med > Volume 16(1); 2021 > Article |
|
Abstract
Background
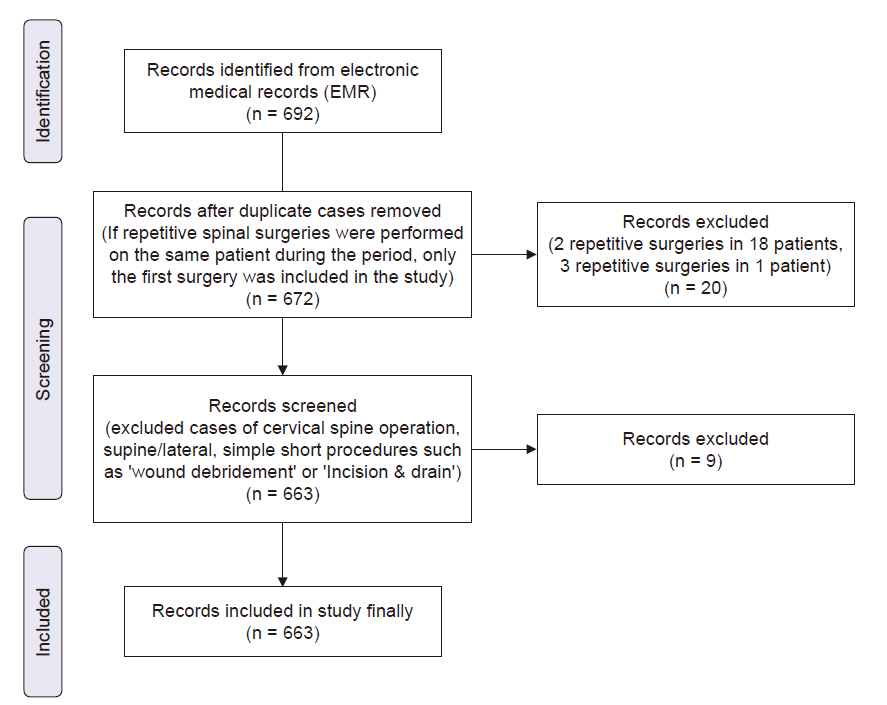
Methods
Results
Conclusions
Notes
Author contributions
Conceptualization: DaeHee Suh, Byunghoon Yoo, Sangseok Lee. Data curation: DaeHee Suh, Sangseok Lee. Formal analysis: Sangseok Lee. Funding acquisition: Sangseok Lee. Methodology: DaeHee Suh, Byunghoon Yoo, Sangseok Lee. Project administration: Sangseok Lee. Writing - original draft: DaeHee Suh, Byunghoon Yoo, Sangseok Lee. Writing - review & editing: Su Yeon Kim, Byunghoon Yoo, Sangseok Lee. Investigation: Su Yeon Kim, Byunghoon Yoo, Sangseok Lee. Software: Sangseok Lee. Supervision: Byunghoon Yoo, Sangseok Lee. Validation: Sangseok Lee.
Table 1.
| Injury area | Stage-1 | Stage-2 | Stage-3 or higher | Total |
|---|---|---|---|---|
| Face | 3 | 11 | 0 | 14 (28.6) |
| Inguinal region | 3 | 11 | 0 | 14 (28.6) |
| Chest | 0 | 12 | 0 | 12 (24.5) |
| ASIS | 1 | 2 | 0 | 3 (6.1) |
| Abdomen | 1 | 2 | 0 | 3 (6.1) |
| Forearm | 0 | 2 | 0 | 2 (4.1) |
| Femur | 1 | 0 | 0 | 1 (2.0) |
| Total | 9 (18) | 40 (82) | 0 | 49 (100)* |
Table 2.
Values are expressed as median (1Q, 3Q), number of patients (%). ASA: American Society of Anesthesiologists, WBC: white blood cell count, PT INR: prothrombin time international normalized ratio, AST: aspartate aminotransferase, ALT: alanine aminotransferase, BUN: blood urea nitrogen, Cr: creatinine.
Table 3.
REFERENCES
-
METRICS

-
- 10 Crossref
- 7,327 View
- 192 Download
- Related articles in Anesth Pain Med
- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others