 |
 |
- Search
| Anesth Pain Med > Volume 16(1); 2021 > Article |
|
Abstract
Background
Transforaminal epidural steroid injection (TFESI) is a conservative treatment for patients with lumbar disc herniation (LDH). However, there are reports of various complications that can occur after TFESI; among these, paraplegia is a serious complication.
Case
A 70-year-old woman who was unable to lie supine due to low back pain exacerbation during back extension underwent TFESI. After injection, there was pain relief and the patient was able to lie supine; however, paraplegia developed immediately. Magnetic resonance imaging confirmed cauda equina syndrome (CES) due to nerve compression from L1-2 LDH. We determined that the patient's LDH was already severe enough to be considered CES and that the TFESI procedure performed without an accurate understanding of the patient's condition aggravated the disease.
Lumbar disc herniation (LDH) commonly causes low back pain (LBP) and radiculopathy. LDH often resolves over time with a spontaneous resorption rate of 60% or above [1]. Therefore, the consensus for treating patients with LDH is to offer conservative treatment first and then surgical intervention for non-responders [2].
One conservative treatment used for LDH is transforaminal epidural steroid injection (TFESI). TFESI is a method used to deliver steroids and local anesthetics into the epidural space through the spinal neural foramen. Numerous reports and extensive reviews have demonstrated the diagnostic efficacy of TFESI as well as its effectiveness in LBP and relief from radiculopathy pain [3]. However, various complications have been reported as the use of TFESI has increased. Some classic complications of TFESI include intravascular injections, vascular trauma, epidural hematoma, and neural damage [4]. There are also case reports documenting paraplegiaŌĆöa serious complicationŌĆöfollowing TFESI. Most of the reported paraplegia cases were due to spinal cord ischemia from a vascular injury or a particulate steroid embolism [5].
We encountered a patient whose severe LBP and radiating pain induced by postural changes prevented the assessment with imaging modalities. Thus, TFESI was performed to relieve the patient's pain. Here, we report this case as the patient developed paraplegia immediately after TFESI.
The patient has provided written informed consent for publication of the case and associated images. This case report follows the CARE (CAse REport) guidelines [6].
A 70-year-old woman came to the emergency room (ER) complaining of severe LBP. The patient was not able to walk, and she was in the left lateral decubitus position with lumbar flexion. The patient's numerical rating scale (NRS, 0 being no pain and 10 being the worst pain imaginable) score for LBP was 6/10, but when asked to perform lumbar extension or move to a supine position, the NRS score for LBP increased to 9/10, with development of left buttock pain and radiating pain in the left thigh. The patient had undergone a posterior lumbar interbody fusion at L2-S1 for a herniated nucleus pulposus 2 years prior to the ER visit. The pain dissipated after the surgery; however, the patient started to experience intermittent recurrences of LBP 1 year after. Three days prior to hospitalization, the patient was unable to lie in the supine position even when sleeping owing to severe LBP and buttock pain.
The patientŌĆÖs height was 154 cm, and her weight was 65 kg. The vital signs included blood pressure 150/90 mmHg, body temperature 36.5┬░C, pulse rate 86/min, and respiratory rate 20/min. Due to the complaint of extreme pain with any change in position, it was necessary to perform a neurological examination on the patient; therefore, the orthopedic surgeon quickly performed the possible tests in the left lateral decubitus position as desired by the patient. However, during the neurological examination, the patient continued to complain of pain. A neurological examination to assess the motor power revealed left ankle dorsiflexion grade 4/5, left big toe dorsiflexion 4/5, left knee extension 4/5, and left hip flexion 4/5, indicating motor weakness. The patient also had a sensory deficit throughout the left leg and complained of numbness in the left thigh. The patient showed an absence of the Babinski reflex, an ankle jerk reflex scale measurement of 2+, and a knee jerk scale measurement of 3+. The right leg did not show any motor weakness or sensory deficit. The patient did not have urinary incontinence or saddle anesthesia, and the anal sphincter tone was retained.
However, we recognized that the patientŌĆÖs spinal disease may be serious due to the patientŌĆÖs history of previous surgery, complaint of severe pain, and abnormal findings on neurological examination of the left lower limb. Consequently, the orthopedic surgeon explained that the disease was severe, and that the patient may require surgery, and additional imaging tests. In our hospital, magnetic resonance imaging (MRI) can only be performed in the supine position; however, as the patient was in a very nervous state due to pain and complained of pain even when moving on the bed or changing position for examination, it was determined that pain control was necessary for additional examination; 100 ŃÄŹ of fentanyl (50 ŃÄŹ/ml) was then administered intravenously. However, the pain relief was inadequate and the patient was unable to change position. Since additional examinations could not be performed, the patient strongly requested priority pain relief before additional imaging examinations.
Our anesthesia and pain medicine department was asked to control this patientŌĆÖs pain. We also considered that the patient may be at high risk for complications with a nerve block because the type of spinal disease was not clearly identified, the state of the nerves could not be ascertained, and abnormal findings were already observed in the neurological examination. However, we understood the urgency of the imaging test; hence, we explained the risk of the procedure and the possibility of side effects to the patient and then planned the pain relief procedure. Given the patientŌĆÖs L2-S1 vertebral body fusion with possible adjacent segment disease, we chose to perform a TFESI through the left L1-2 neural foramen.
The procedure was conducted 3 h after the patient arrived at the ER, with the patient kept in her preferred left lateral decubitus position with lumbar flexion. C-arm fluoroscopy was performed, and the typical lateral view angle was used in order to obtain the anteroposterior view. A fluoroscopic lateral image indicated a kyphotic deformity at the L1 vertebral body, likely caused by osteonecrosis.
After the skin had been sterilized, 2 ml of 1% lidocaine was administered for local anesthesia. To create an oblique view in order to visualize the left L1-2 neural foramen, the C-arm angle was turned 20┬║ to the left from the anteroposterior view. A 20-gauge short bevel nerve block needle was inserted until the needle tip reached the inferior margin of the L1 lumbar pedicle, and the lateral view was checked after the needle tip reached the middle of the pedicle. In the lateral view, the needle tip was located immediately before reaching the dorsal periosteum of the L1 vertebral body; 2 ml of contrasting agent was used to confirm that the location of the needle tip was appropriate for the epidural injection (Fig. 1). No blood vessel contrasting was observed. During the procedure, the contrast agent did not disappear rapidly due to blood or cerebrospinal fluid flow. The contrast agent showed a pattern of spreading along the epidural space.
A 6 ml mixture containing 10 mg of 0.5% bupivacaine (5 mg/ml), 3 ml of normal saline, and 5 mg of dexamethasone (5 mg/ml) was injected slowly.
Five minutes after the injection, the patientŌĆÖs LBP NRS score decreased to 2/10. A neurological examination showed no change in motor or sensory functions compared to pre-injection. Her vital signs were as follows: blood pressure, 124/68 mmHg; body temperature, 36.5┬░C; pulse rate, 70/min; and respiratory rate, 16/min. Although the blood pressure was lower than that before the procedure, it was within the normal range, and this was judged to be due to the reduction in pain. When epidural nerve block is performed, neurological changes and changes in vital signs may occur slowly, and thus additional patient monitoring was necessary. However, after further discussions with an orthopedic surgeon, it was decided that the imaging test should be performed quickly. The patient was able to lie supine with reduced pain and was sent for an MRI. During the MRI scan, the patient reported acute paraplegia and a complete loss of motor and sensory functions in both legs including the sensation around the anus. The patient also lost the anal reflex and bulbocavernosus reflex. The vital signs were as follows: blood pressure, 118/70 mmHg; body temperature, 36.6┬░C; pulse rate, 70/min; and respiratory rate, 18/min.
We assessed the situation at the time of the procedure, and contrasted images were reviewed to determine the cause of paraplegia. The operator who had performed TFESI judged that the contrast medium had spread to the epidural space. The possibility of intrathecal injection could not be completely ruled out. However, we injected bupivacaine at a low concentration, so the complete loss of motor sensory function as seen in this patient was determined to be unlikely. We also speculated that the progress of cauda equina syndrome (CES) may have been accelerated due to the effect of the pressure or volume when the drug was injected. It was also impossible to completely rule out the possibility of a hematoma being produced due to blood vessel damage caused by the needle. We could obtain the patientŌĆÖs MRI results. Upon assessing the MR images, we found that the patientŌĆÖs conus medullaris was located above the L1 body and CES occurred due to L1-2 LDH. There was no indication of any cord injury (Fig. 2).
An emergency decompression surgery was performed 1 h after the paraplegia developed. The L1 lamina was excised, and decompression and discectomy on both sides were performed. In order to resolve the kyphotic deformity, posterior lumbar fusion was also performed at T11-L1 (Fig. 3). The surgeon confirmed that the dural sac was strongly compressed by the disc at the L1-2 level during surgery. In addition, there was slight bleeding around the disc at L1-2. The situation was determined to be inconsistent with nerve compression due to bleeding. However, it was difficult to clearly identify the cause of this bleeding. It was not possible to specify whether bleeding occurred due to the TFESI procedure, or whether blood vessel damage occurred due to pressure applied to the inner portion of the spinal canal by the disc.
The patientŌĆÖs paralysis did not resolve after the surgery. The patient was hospitalized for 6 weeks, and repeated neurological examinations were conducted to assess signs of recovery. At week 6, no change in motor function, and only a mild recovery of sensory function were observed. There was a recovery of fine touch and proprioception in both thighs, but the bladder function did not recover. The patient was transferred to a rehabilitation hospital at her request, and she agreed to come in for a 6-month follow-up. At the follow-up visit, the patient had a 3/5 of muscle strength grade and did not have any voiding difficulties. However, numbness throughout both legs persisted.
Recently, TFESI has been widely used in patients with various spinal diseases. TFESI is particularly useful for pain relief in patients with LDH. However, several complications caused by TFESI, including infection, vascular injury, hematoma, intravascular drug injection, nerve damage, embolism, and paraplegia, have been reported. To prevent the occurrence of these complications, we need to understand the patient's disease state as early as possible and decide on the most appropriate treatment plan. The process of making this judgment is facilitated by the patient's medical history, neurological examination, and imaging tests. Among these, the most helpful information is provided by the MRI examination [2].
It is very rare to encounter a patient whose posture change is completely impossible due to extreme pain, as was the case with our patient. As a result, the patient was unable to lie in a supine position, making imaging tests completely impossible. In general, if a patient's symptoms are severe and neurologic deficit is involved, imaging tests are performed first. TFESI is then performed to facilitate the diagnosis and treatment of the patient. However, we were asked to perform a TFESI for the purpose of performing an imaging test without being provided with any imaging test results prior to the procedure. The patient's pain was not controlled even with narcotic analgesics. The initial neurological examination did not prompt us to suspect CES. Under the opinion that the MRI was necessary even for surgery, we proceeded with TFESI. At that time, the patient complained of extreme pain and had abnormal neurological examination findings. If a patient shows CES or neurological symptoms are progressing rapidly, surgical treatment should be selected [2].
According to the results of the MRI, which was performed after TFESI and the patient's pain had been alleviated, it was presumed that the patient had already had K├╝mmellŌĆÖs disease or spondylodiscitis. Further, a kyphotic deformity due to osteonecrosis was progressing at the L1-2 level. In addition, severe LDH of L1-2 could cause severe pain and paralysis. It was presumed that the patient was avoiding paralysis by keeping the spinal canal wide via lumbar flexion [7]. So, it seems that the patient felt severe pain and refused to adopt a position of back extension. This patient developed paraplegia after TFESI, as the disease rapidly worsened. The relationship between CES and TFESI in this patient is not clear. However, there are several possible causes that may have led to CES in this patient.
First, CES may have occurred due to a rapid increase in pressure within the epidural space as the drug was injected during the procedure. According to a study by Usubiaga et al. [8], pressure in the epidural space can increase from -10 cmH2O to a maximum of 65 cmH2O when 10 ml of 2% lidocaine is injected. In particular, pressure in the epidural space was higher in elderly patients, and high levels of pressure could be maintained up to 2 min after injection of the drug. The patient in our case had an epidural space volume that was too small for her to tolerate pain without adopting the lumbar flexion position. For this reason, it was thought that the pressure created by drug injection into the epidural space acted more strongly. If such an elderly patient is expected to have high pressure in the epidural space due to severe LDH, a small amount of the drug should be injected as slowly as possible.
Alternatively, it is possible that blood vessel damage occurred. The radicular artery enters the intervertebral foramen along the nerve root. The probability of the radicular artery being in the upper portion of the intervertebral foramen is twice as high as that of it being in the lower portion [9]. The patient in our case had undergone posterior lumbar interbody fusion surgery at the L2-S1 level, and it was assumed that severe LDH occurred at the L1-2 level. We predicted that it would be difficult for the needle to enter the lower portion of the foramen while performing TFESI at the L1-2 level and instead inserted the needle into the upper portion of the foramen. Although the blood vessels were not imaged using a contrast agent, the possibility that blood vessel damage occurred cannot be excluded. In addition, the radicular artery or internal vertebral venous plexus may have been damaged as the pressure in the epidural space increased as mentioned previously [10]. It is possible that this vascular injury contributed to the occurrence of CES.
A third reason, post-procedural changes in posture due to pain relief may have exacerbated the disease. The lumbar flexion posture can exacerbate LDH by applying pressure within the disc. However, the lumbar flexion position increases the capacity of the spinal canal [7]. As mentioned previously, the patient had already experienced a serious LDH condition that caused CES, but her position may have reduced the pressure applied to the dural sac by increasing the diameter of the spinal canal with lumbar flexion. However, after TFESI, the patient was able to lie in the supine position because back extension was possible. At this time, the capacity of the spinal canal would have decreased. As a result, it is expected that the dural sac was strongly pressed and CES occurred immediately. There is an existing case report of CES that progressed according to a similar mechanism [11]. In the reported case, the patient was diagnosed with spinal stenosis, and an MRI scan was difficult due to the severe pain experienced by the patient when in the supine and back extension position. Hence, to proceed with the examination, the patient was sedated with propofol while lying in the supine position. Subsequently, an MRI scan was performed and CES occurred.
Before TFESI is performed, it is important to determine the patient's neurological condition, disease, and cause of pain via MRI. However, as was observed in our case, if a posture change is impossible and the imaging test cannot be performed, it can be challenging to effectively treat the patient. Recently, MRI equipment capable of performing examinations in various postures such as sitting or standing has been developed and used [12]. The use of such equipment is thought to be helpful for imaging tests in patients who are unable to maintain a supine position due to pain.
However, if such equipment is unavailable, the cause and severity of pain, as well as the risk of the procedure, should be determined by reviewing the patient's medical history and performing a neurological examination. In patients with LDH, lumbar motion limitation, resting pain, and deformity are red flags [13]. In addition, patients who experience leg pain during lumbar extension have a poor prognosis [14]. When TFESI is performed on high-risk LDH patients, a thorough assessment of position- and motion-based pain characteristics, including a neurological examination, is necessary. Patients should be informed and educated about the risks of exacerbation of their existing disease with positional changes after pain relief from TFESI. In addition, when performing TFESI on high-risk LDH patients, physicians should be prepared for any emergency.
In our case, the patient developed CES due to L1-2 level LDH. Fortunately, the patientŌĆÖs conus medullaris was located above the L1 body; however, the conus medullaris is usually located between T12 and L2. Conventionally, if the dural sac of the L1-2 level is compressed, not only CES but also conus medullaris syndrome (CMS) can occur. In both CES and CMS, radiating pain, as well as motor and sensory dysfunction of the lower extremities, can occur, and bladder dysfunction and saddle anesthesia may be seen. Since both syndromes show similar symptoms, it is difficult to distinguish them based on clinical features alone; however, they are easily distinguishable via MRI. Additionally, treatment of both syndromes commonly requires emergency decompression surgery [15]. If CMS would have occurred, recovery would have been more difficult even if emergency decompression surgery had been performed.
We performed TFESI without an accurate initial assessment of the patient's disease state and observed paraplegia in this patient after TFESI had been performed. It is important to accurately evaluate the patient before this procedure, establish a correct treatment plan, and safely perform the procedure using methods designed to reduce the occurrence of complications. In addition, it is important to explain the risks and possible complications of the procedure to the patient, so that they are able to prepare for the possibility of experiencing serious complications. Even when extreme care is taken, complications may occur after the procedure. If such a complication occurs, the rapid identification of its cause and a prompt response greatly affect patient recovery.
Fig.┬Ā1.
(A) Anteroposterior view fluoroscopy shows the needle, which was inserted under the L1 lumbar pedicle. (B) Lateral view fluoroscopy shows the contrasting agent spreading from the L1-2 neural foramen into the epidural space.
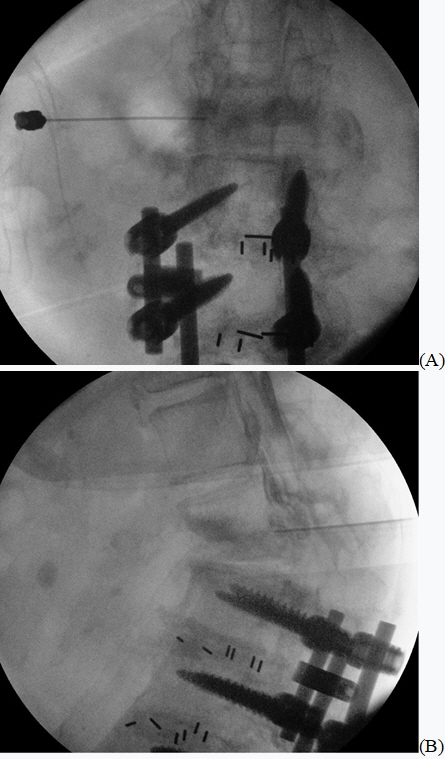
Fig.┬Ā2.
(A) Lumbar sagittal T2-weighted magnetic resonance imaging shows compression of the cauda equina due to L1-2 lumbar disc herniation (white arrow). Conus medullaris (black arrow). (B) Lumbar axial T2-weighted magnetic resonance image showing compression of the cauda equina due to L1-2 lumbar disc herniation.

REFERENCES
1. Zhong M, Liu JT, Jiang H, Mo W, Yu PF, Li XC, et al. Incidence of spontaneous resorption of lumbar disc herniation: a meta-analysis. Pain Physician 2017; 20: E45-52.

2. Andersson GB, Brown MD, Dvorak J, Herzog RJ, Kambin P, Malter A, et al. Consensus summary of the diagnosis and treatment of lumbar disc herniation. Spine (Phila Pa 1976) 1996; 21(24 Suppl): 75S-8S.


3. Vad VB, Bhat AL, Lutz GE, Cammisa F. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine (Phila Pa 1976) 2002; 27: 11-6.


4. Boswell MV, Shah RV, Everett CR, Sehgal N, McKenzie Brown AM, Abdi S, et al. Interventional techniques in the management of chronic spinal pain: evidence-based practice guidelines. Pain Physician 2005; 8: 1-47.


5. Kennedy DJ, Dreyfuss P, Aprill CN, Bogduk N. Paraplegia following image-guided transforaminal lumbar spine epidural steroid injection: two case reports. Pain Med 2009; 10: 1389-94.



6. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol 2014; 67: 46-51.


7. Dai LY, Xu YK, Zhang WM, Zhou ZH. The effect of flexion-extension motion of the lumbar spine on the capacity of the spinal canal. An experimental study. Spine (Phila Pa 1976) 1989; 14: 523-5.


8. Usubiaga JE, Wikinski JA, Usubiaga LE. Epidural pressure and its relation to spread of anesthetic solutions in epidural space. Anesth Analg 1967; 46: 440-6.

9. Melissano G, Chiesa R. Advances in imaging of the spinal cord vascular supply and its relationship with paraplegia after aortic interventions. A review. Eur J Vasc Endovasc Surg 2009; 38: 567-77.


10. Desai MJ, Dua S. Perineural hematoma following lumbar transforaminal steroid injection causing acute-on-chronic lumbar radiculopathy: a case report. Pain Pract 2014; 14: 271-7.


11. Kuramoto A, Chang L, Graham J, Holmes S. Lumbar spinal stenosis with exacerbation of back pain with extension: a potential contraindication for supine MRI with sedation. J Neuroimaging 2011; 21: 92-4.



12. Jinkins JR, Dworkin JS, Damadian RV. Upright, weight-bearing, dynamic-kinetic MRI of the spine: initial results. Eur Radiol 2005; 15: 1815-25.



13. Verhagen AP, Downie A, Popal N, Maher C, Koes BW. Red flags presented in current low back pain guidelines: a review. Eur Spine J 2016; 25: 2788-802.



-
METRICS

-
- 2 Crossref
- 6,754 View
- 111 Download
- Related articles in Anesth Pain Med
-
Spinal intramedullary cavernous angioma patient in a pain clinic - A case report -2022 October;17(4)
Liver transplantation of a patient with extreme thrombocytopenia - A case report -2021 July;16(3)
- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others








