Awake craniotomy has become a feasible treatment option for patients with brain tumors residing within or close to regions presumed to have language or sensorimotor functions [
1]. Different anesthetic techniques, including conscious sedation with monitored anesthesia care (MAC) and the asleep-awake-asleep technique, have been applied for awake craniotomy [
2]. However, in all these techniques, respiratory depression, including desaturation and hypercapnia during surgery, has been reported as an important complication [
3].
Various techniques have been introduced to reduce the risk of respiratory insufficiency. The use of a high-flow nasal cannula (HFNC) can improve oxygenation compared to conventional oxygen devices by reducing the anatomical dead space, producing positive airway pressure, and facilitating the clearance of carbon dioxide in patients with spontaneous respiration [
4]. Moreover, HFNC with a humidifier and heated circuit can deliver a conditioned gas mixture through a nasal cannula at up to 60 L/min with a fraction of inspired oxygen (FiO
2) ranging from 0.21 to 1 for better patient comfort [
3]. On the other hand, the oxygen reserve index (ORi), a tool for measuring the oxygen reserve, is reportedly useful for the early detection of hypoxia [
5,
6]. The measurement of partial pressure of oxygen (PaO
2) by atrial blood gas analysis is intermittent and requires invasive techniques. Pulse oximetry saturation (SpO
2) can detect hypoxia, but responds slowly and may not significantly decrease until the PaO
2 is below 80 mmHg [
7]. ORi is a unitless index from zero to 1, which correlates with PaO
2. A sharp decrease in ORi or a drop to zero means that the oxygen reserve is low, and hypoxia is expected soon. This continuous and noninvasive technique enables early warning to prevent desaturation. We report two cases of patients who underwent moderate to conscious sedation for awake craniotomy. In two cases, a combination of HFNC and ORi was used to prevent respiratory insufficiency. Our Institutional Review Board (no. SMC 2020-11-109) approved this study and waived the requirement for written informed consent.
CASE REPORT
Case 1
The patient was a 58-year-old male with glioblastoma located near Broca's area. Brain functional magnetic resonance imaging showed that the motor and sensory cortices were located anterior to the mass. To preserve language and motor function, an awake craniotomy was scheduled [
1]. Preoperative physical examination with an anesthetic evaluation, including the airway, was performed. The patient was taking medication for dyslipidemia, and his cardiopulmonary function was normal. His mallampati score was 2, and there were no abnormalities in the anatomy of the airway. The body mass index (BMI) was 26.5 kg/m
2, and the patient reported that he snores heavily when sleeping. To assess obstructive sleep apnea (OSA), the STOP-BANG (acronym for Snoring, Tiredness, Observed apnea, high blood Pressure, Body mass index, Age, Neck circumference, and Gender) questionnaire was used, based on which he was classified as having a high risk of OSA. We applied the HFNC (AIRVO 2 system, Fisher & Paykel, New Zealand) to improve oxygenation during surgery [
4]. EtCO
2 was monitored using a commercial sample line provided with the AIRVO 2 system. In addition, we monitored ORi to measure the oxygen reserve and the effectiveness of HFNC.
We used standard monitors (electrocardiogram, noninvasive blood pressure, end-tidal carbon dioxide, and pulse oximeter) with a bispectral index (BIS VISTA, Aspect Medical Systems, USA) sensor and administered 4 mg of ondansetron, 0.2 mg of glycopyrrolate, and 1 mg of midazolam as premedication. The scalp nerve block (a perineural injection of 0.75% ropivacaine [26 ml] mixed with 1:200,000 epinephrine) was performed by an experienced anesthesiologist to block the supraorbital, supratrochlear, auriculotemporal, zygomaticotemporal, lesser occipital, and greater occipital nerves [
8]. After initiating MAC with propofol and remifentanil, radial arterial cannulation was performed for invasive arterial monitoring, followed by insertion of a peripheral intravenous cannula and Foley catheter. For effect-site target-controlled infusion (TCI) for MAC, a commercial TCI pump (Orchestra Base Primea, Fresinus Vial, France) was used, and the pharmacokinetic set used to calculate the target effect site concentration (Ce) was the Minto model for remifentanil and Schneider model for propofol [
2]. Usually, the target BIS is 60-70 during sedation for craniotomy [
4], but in this case, we targeted the BIS above 70 to reduce the potential risk of airway obstruction following deep sedation. We applied both HFNC and ORi at the beginning of sedation and adjusted the flow and FiO
2 of HFNC when ORi dropped to nearly zero, which is an early indicator of hypoxia.
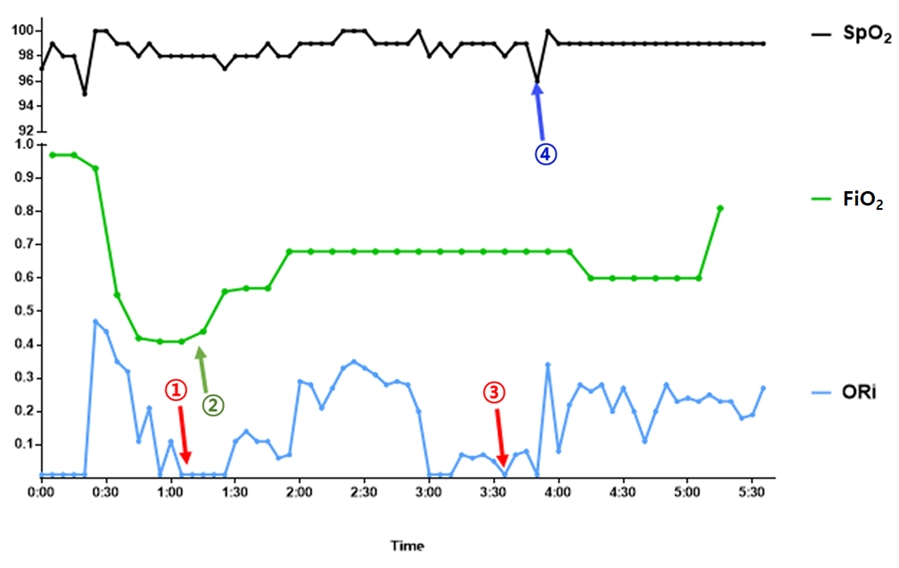
Fig. 1 shows the values of ORi, FiO
2 of HFNC, and SpO
2 during surgery. At the beginning of sedation, HFNC was applied at 15 L/min and FiO
2 at 0.4. At this time, the PaO
2 and SpO
2 were 118.3 mmHg and 100%, respectively. When we increased the target Ce of remifentanil before the expected stimulation, such as head fixation and incision, a sudden decrease in ORi to zero occurred, but ORi was recovered by increasing FiO
2. During the speech test, ORi dropped to zero. Although oxygen saturation was maintained at 100%, ORi remained at zero even when the flow of HFNC increased from 15 L/min to 30 L/min. As he was almost awake, the possibility of airway obstruction was low; therefore, we checked the HFNC machine and found that the nasal cannula was pulled out while the patient was speaking. When the nasal cannula was applied to the patient again, the ORi recovered above 0.2. When ORi was zero, SpO
2 was 100%, but dropped to 95% transiently after ORi recovered. During the speech test, the ORi repeatedly dropped to zero, confirming that the nasal cannula was removed from the patient. After the neurologic examination, ORi was maintained above 0.2 with FiO
2 at 0.6 and a flow rate of 30 L/min with HFNC until the end of the surgery (
Table 1). The estimated blood loss was 300 ml, heart rate was approximately 70 beats per minute, and mean arterial blood pressure was maintained above 70 mmHg during the surgery. The patient underwent successful awake craniotomy and tumor resection without any loss of neurological function or perioperative adverse events.
Case 2
The patient was a 33-year-old male with a low-grade glioma located in the left basal ganglia near the inferomedial aspect of BrocaŌĆÖs area. He had smoked for 14 years, and his cardiopulmonary function was within normal limits. His BMI was 25.3 kg/m2, and physical examination revealed a mallampati score of 2 and no evidence of airway abnormality or signs of OSA with normal PaO2 before surgery. Anesthesia was performed in the same order as described in the previous case. The scalp nerve was blocked using 30 ml of 0.75% ropivacaine with 1:200,000 epinephrine. We applied HFNC at 30 L/min and FiO2 at 0.5, with ORi monitoring. After sedation, the PaO2 was 387.3 mmHg under HFNC. The patient was sedated with a target BIS of 60-70. The ORi value was recorded throughout the surgery.
After the initiation of sedation, the ORi suddenly decreased to zero; therefore, we increased the FiO2 to 0.77. Five minutes later, the ORi was restored to 0.29; accordingly, the FiO2 was changed to 0.5. During the surgery, the ORi suddenly decreased again from 0.45 to 0.2, for which the FiO2 was increased briefly to 0.77 before reducing it back to 0.5 after recovery of ORi. Oxygen saturation remained at 100%, while ORi changed from 0 to 0.85 throughout the surgery. During surgery, sedation and awake procedures were performed. While the ORi tended to increase temporarily when the patient woke up, there was no significant relationship between changes in ORi and patient consciousness.
The patient underwent extensive sensory and motor testing as well as neurocognitive testing, including naming, reading, counting, and verbal fluency. The total estimated blood loss was 300 ml, heart rate was 90 to 100 beats per minute, and mean arterial blood pressure was above 80 mmHg, which was similar to that before surgery. TCI sedative agents and HFNC regimen were recorded (
Table 2). The patient underwent successful awake craniotomy and tumor resection without any loss of neurological function or perioperative adverse events.
DISCUSSION
In these two cases, the patients underwent MAC during awake craniotomy. They were awake during the neurological examination, but remained sedated during the rest of the procedure. We used propofol and remifentanil as sedatives [
2], which may induce respiratory insufficiency in a dose-dependent manner. In particular, in patients with OSA, even a small increase in the sedative dose may cause unexpected respiratory insufficiency; therefore, the dose of the sedative was adjusted under close monitoring of BIS. Airway obstruction due to respiratory insufficiency can cause intracranial hypertension [
4]; therefore prompt management is required. However, securing the airway with laryngeal mask airway insertion or endotracheal intubation is challenging in patients undergoing awake craniotomy because of unusual positions and head fixation, which may delay prompt airway intervention and lead to serious consequences during surgery.
To prevent respiratory insufficiency, we attempted to improve oxygenation by applying HFNC. MAC with HFNC is a reasonable option for patients undergoing awake craniotomy, especially for those with OSA [
4]. A high flow of oxygen is supplied to generate resistance to expiratory flow, resulting in a continuous positive airway pressure effect. According to the other study, when HFNC was applied at 35 L/min, positive pressures produced 2.7 cmH
2O with the mouth open and 1.2 cmH
2O with the mouth closed, and the difference was more pronounced when the BMI was higher [
9]. In addition, carbon dioxide is easily released and the dead space is decreased, which is effective in resolving dyspnea [
10]. In addition, humidified gas improves mucociliary function, helps secretion clearance, and reduces atelectasis [
4]. These advantages of HFNC are particularly useful in patients with OSA undergoing awake craniotomy.
ORi is a noninvasive and continuous supplemental tool that can provide early warning to clinicians regarding changes in a patientŌĆÖs oxygen reserve. It is a unitless index between 0 and 1, where 0 indicates a decrease in PaO
2 and impending hypoxia [
5,
11]. In the study of ORi and PaO
2, they were significantly correlated; moreover, a decrease in ORi to approximately 0.24 may provide an advanced indication of decreasing PaO
2 [
11]. The ORi was reported to detect an impending desaturation at a median duration of 31.5 s before any noticeable changes in SpO
2 occurs during surgery in children [
12]. In the first case, ORi decreased several times but was recovered by slightly increasing the FiO
2 of the HFNC. During neurological examination, ORi detected hypoxia faster than SpO
2. The ORi dropped to zero while the HFNC was pulled out, but SpO
2 remained at 100%. After reapplying HFNC, ORi was maintained above 0.2, but SpO
2 dropped to 95% only and recovered soon, indicating that the change in ORi was faster than that of SpO
2. At that time, we could not measure the exact value of PaO
2 by arterial blood gas analysis, but if we did not measure the ORi, it would have been late to find out that HFNC was pulled out and respiratory insufficiency would have occurred. Although the patient was awake, remifentanil was continuously infused during the neurological examination; therefore, he could easily experience hypoxia. ORi allows real-time surveillance of the oxygenation status and may enable proactive interventions to avoid hypoxia. In the second case, we adjusted the FiO
2 of the HFNC when ORi decreased; thus, SpO
2 remained at 100%. The flow rate of HFNC was maintained at 30 L/min from the beginning, but as in the first case, the oxygen reserve could be properly maintained by adjusting the flow rate of the HFNC. HFNC helps to prevent hypoxia while preventing airway obstruction by controlling FiO
2 to values from 0.2 to 1 and providing a positive end-expiratory pressure effect. Although it is still controversial, since hyperoxia might have a negative effect on craniotomy [
13], it is recommended to maintain normoxia during and after craniotomy [
14]. Hyperoxia could occur due to HFNC; thus, decreasing the FiO
2 when the ORi approaches 1 might help to maintain normoxia.
In conclusion, the combination of HFNC and ORi monitoring could provide adequate oxygen reserve in patients while effectively preventing a decrease in oxygen saturation during awake craniotomy.