Comparison of nebulized dexmedetomidine and ketamine for premedication in pediatric patients undergoing hernia repair surgery: a randomized comparative trial
Article information
Abstract
Background
Allaying anxiety and providing calm children in the operating room is a challenging task for anesthesiologists. This study was designed to compare the use of nebulized dexmedetomidine and ketamine for premedication in pediatric patients under general anesthesia.
Methods
Seventy patients, aged 2 to 8 years of both sexes, with American Society of Anesthesiologists physical status I/II scheduled for hernia repair surgery under general anesthesia, were randomized to two equal groups using a computer-generated random number table. Patients in group D received dexmedetomidine (2 µg/kg), and patients in group K received ketamine (2 mg/kg) by a jet nebulizer before the induction of anesthesia. The study's primary objective was comparing the level of sedation, which was achieved at 30 min after a study drug administration using the Ramsay sedation scale, between the two groups. The secondary objectives were the two-group comparison of parental separation anxiety scale, acceptance of the mask, hemodynamic variables, recovery time, incidence of emergence agitation, and adverse events.
Results
The median Ramsay sedation scale at 30 min was 3 (1–4) in group D and 3 (1–3) in group K (P = 0.002). Patients in group D had a more acceptable parental separation anxiety scale (P = 0.001) and a satisfactory mask acceptance scale (P = 0.042).
Conclusions
Nebulized dexmedetomidine (2 µg/kg) provided better sedation along with smooth parental separation and satisfactory mask acceptance during induction of anesthesia with a similar emergence agitation profile and adverse reactions compared to nebulized ketamine in pediatric patients.
INTRODUCTION
For decades, there has been a quest to look out for a reliable and efficacious premedication to allay anxiety and fear of the stressful preoperative period. The leading factors contributing to preoperative anxiety in pediatric patients are parental separation, fear of doctors, needle injections, limited understanding of the nature of the illness, and the need for surgery [1]. Preoperative anxiety and stress responses are responsible for the activation of the sympathetic, parasympathetic, and endocrine systems. Children are likely to develop adverse clinical outcomes such as postoperative psychological trauma, emergence delirium, changes in sleep patterns, and aggression [2,3]. The reduction in preoperative anxiety and distress in children is not only an ethical imperative but also helpful in minimizing postoperative behavioral problems with the aid of a suitable premedication.
An ideal premedication drug should result in a sedated child to allow easy separation of a child from the parents, facilitating smooth induction of anesthesia and a pleasant perioperative experience for both children and parents. Although many studies have reported the effects of benzodiazepines, α-2 agonists, opioids, and ketamine as premedication drugs via various routes, there is no widely accepted drug or route of choice [4–6]. Most of these drugs produce variable sedation, with a risk of respiratory depression. Studies have reported higher bioavailability and fewer adverse events with the nebulized route than with oral or intranasal administration [7–10].
A few studies were available in the literature that compared the nebulized dexmedetomidine and ketamine as a premedication in pediatric patients undergoing inguinal hernia surgery under general anesthesia [11–13]. The hypothesis of this study was that nebulized dexmedetomidine and ketamine as premedications are equally effective in terms of sedation at 30 min in pediatric patients. The primary objective of this study was to assess the level of sedation achieved after 30 min of nebulization and to compare between the two groups, and the secondary objectives were parental separation anxiety scale, mask acceptance scale, recovery time, emergence agitation, hemodynamic changes, and adverse effects.
MATERIALS AND METHODS
Study design
The study was approved by the Institutional Ethical Committee (no. SNMC/IEC/2019/Plan/180) and registered at the Clinical Trial Registry of India (no. CTRI/2019/11/022270) before the commencement of the study. This clinical study was performed in accordance with the ethical principles for medical research involving human subjects outlined in the Helsinki Declaration of 1975 (revised 2013). This prospective, randomized study was conducted at a tertiary care teaching hospital between December 2019 and November 2020, after obtaining written informed consent from the parents or guardians. Preoperative evaluation of all patients was carried out one day before the scheduled surgery.
Study population and interventions
1. Participants and eligibility
A total of 70 patients, aged 2–8 years of both sexes, American Society of Anesthesiologists physical status I and II, who underwent hernia repair surgery under general anesthesia (surgical duration less than 60 min), were enrolled in this study.
2. Exclusion criteria
Patients with known allergy to the study drug, upper respiratory tract infection, any nasal disorders such as recurrent nasal bleed or nasal masses, or congenital heart disease, children with increased intracranial pressure/intraocular pressure, and children with any psychiatric illness were excluded from the study.
3. Primary objective
The primary objective of the study was to examine the level of sedation achieved at 30 min using the Ramsay sedation scale (RSS).
4. Secondary objectives
The secondary objectives were parental separation anxiety scale, acceptance of the mask, hemodynamic variables, recovery time, incidence of emergence agitation, and adverse events.
5. Randomization
Patients were randomly divided into group D (nebulized dexmedetomidine 2 µg/kg, 35 patients) and group K (nebulized ketamine, 2 mg/kg, 35 patients) using a computer-generated random number table.
6. Allocation concealment
The group concealment was performed with a sealed opaque envelope by an independent investigator who was not involved in the study. The envelope was opened immediately after the arrival of a child in the preoperative holding area. The drug solution was prepared by an anesthesiologist, who was not involved in the observation or administration of the drug in identical syringes with matching random codes. The study drug was diluted in 4 ml by adding 0.9% normal saline in both groups.
7. Blinding
The observers, attending anesthesiologists, and data collectors were blinded to the drug being administered.
8. Monitoring
Standard American Society of Anesthesiologists monitors such as an electrocardiogram, non-invasive blood pressure, and pulse oximetry were applied, and baseline heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure, mean arterial blood pressure, respiratory rate, and peripheral oxygen saturation were recorded.
9. Intervention
The study drug was administered by a standard hospital jet nebulizer (Romsons Aero Neb nebulizer cup and mask set, Romsons Scientific & Surgical Pvt. Ltd., India) with an oxygen flow of 6 L/min over 10 to 15 min. Nebulization was stopped when the nebulizer started to sputter.
Outcomes
The sedation scale was assessed using the RSS immediately after completion of drug administration (0 min) and every 5 min for 30 min. A score of 3 or 4 was considered acceptable for sedation (Table 1) [14].
Parental separation was assessed 30 min after completion of study drug administration using a four-point parental separation anxiety scale (PSAS) (1 = excellent, easy separation; 2 = good, whimpers, but is easily reassured, not clinging; 3 = fair, cries and cannot be easily reassured, but not clinging to parents; and 4 = poor, crying, and clinging to parents) [15]. PSAS scores of 1 and 2 were considered acceptable separation, while scores of 3 and 4 were considered difficult separation.
Patients’ acceptance was assessed 30 min after completion of study drug administration by mask acceptance scale (MAS) (1 = excellent, unafraid, cooperative, accepts mask easily; 2 = good, slight fear of mask, easily assured; 3 = fair, moderate fear of mask, not calmed with reassurance; 4 = poor, terrified, crying, or combative) [15]. The MAS scores of 1 and 2 denote “satisfactory” mask acceptance, whereas scores of 3 and 4 were considered “unsatisfactory.”
Hemodynamic parameters were recorded after completion of drug administration (0 min) and every 5 min for 30 min.
Anesthesia technique
Anesthesia was induced with 100% oxygen and sevoflurane 8% with the help of a face mask. After loss of consciousness, an intravenous line was secured on the dorsum of the hand, and fentanyl 2 µg/kg intravenously (IV) was administered. The airway was secured using an appropriately sized laryngeal mask airway (LMA). Anesthesia was maintained by a minimum alveolar concentration of 1–1.2% of sevoflurane in a 50% oxygen/air mixture. Paracetamol (15 mg/kg IV) was administered to all patients before the completion of surgery. Upon regaining consciousness, the LMA was removed, and the patient shifted to the post-anesthesia care unit. Emergence agitation (EA) was assessed immediately after removal of LMA (0 min) and every 5 min thereafter until 30 min using the Watcha scale [16].
Anesthesia time (from the time of the start of induction to the time of discontinuation of sevoflurane), surgical time (from the time of incision to the application of last skin suture), and recovery time (from the time of discontinuation of sevoflurane to the time the patient opened his/her eyes on verbal command) were recorded. A fall in HR < 70 beats/min and a fall in SBP > 20% from basal level were considered bradycardia and hypotension, respectively, and managed accordingly.
Other adverse effects, such as nausea, vomiting, and hypersalivation, were documented and managed according to the standard protocol.
Sample size calculation
The sample size was calculated based on a previous study [11]. The proportion of patients who achieved adequate sedation at 30 min after drug administration was 46% in the dexmedetomidine group and 13% in the ketamine group. Considering 80% power and 95% confidence intervals, a minimum sample size of 30 children per study group was estimated. To round off and cover the possible dropouts, 35 children in each group were included.
Statistical analysis
The data obtained were entered into a Microsoft spreadsheet and analyzed using Statistical Package for Social Science (SPSS) version 24.0 (IBM Co., USA). The normal distribution of data was confirmed using the Shapiro–Wilk test. It showed that our data were normally distributed, and thus, parametric tests have been used for statistical analysis. The unpaired Student t-test was used for quantitative variables (hemodynamic parameters), and the chi-square test (sedation level, PSAS) and Fisher exact test (emergence agitation, MAS) were used for qualitative variables. Continuous variables are expressed as mean ± SD, and categorical data are presented as numbers and frequencies. A one-way repeated measures ANOVA was conducted to identify significant changes in vital parameters at different time periods. Statistical significance was set at P < 0.05.
RESULTS
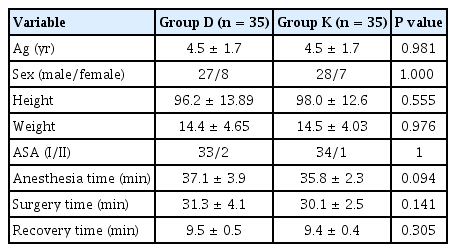
A total of 75 children were recruited for the trial. The parents of two patients did not provide consent for participation in the study, and three patients with upper respiratory tract infection were excluded from the study. The remaining 70 patients were randomly divided into two equal groups of 35 patients each (Fig. 1). The demographic data, surgical time, anesthesia time, and recovery time were not statistically significant between the two groups (Table 2).

CONSORT flow diagram. CONSORT: consolidated standards of reporting trials, ASA PS: American Society of Anesthesiologists physical status, URTI: upper respiratory tract infection.
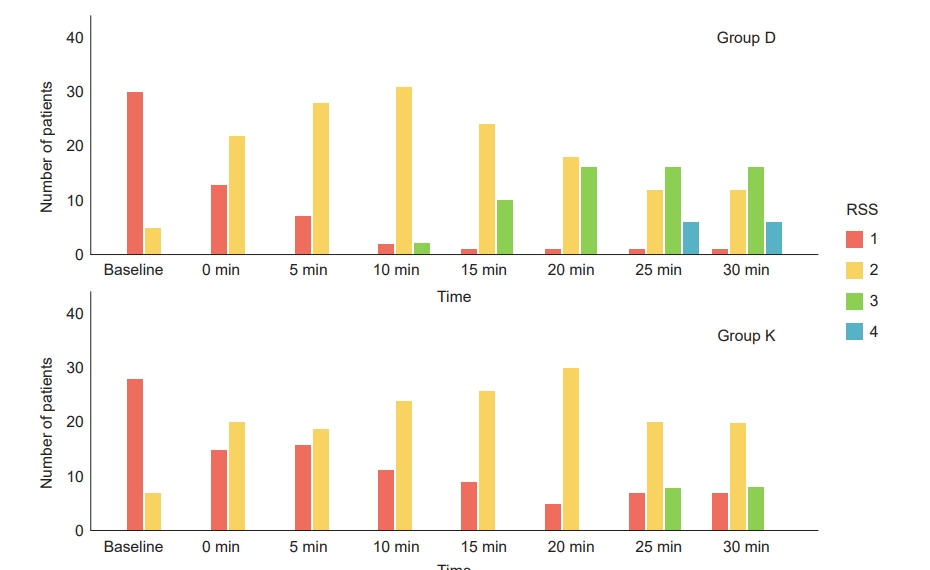
The median Ramsay sedation scale at 30 min was 3 (1–4) in group D and 3 (1–3) in group K (P = 0.002) (Table 3). At 30 min, 16 (45.7%) patients in group D and 8 (22.8%) patients in group K achieved an RSS of 3 (P = 0.002), whereas RSS 4 was achieved in 6 (17.1%) patients in group D and none in group K (Fig. 2). The parental separation anxiety was within the acceptable range, and mask acceptance was satisfactory in group D compared to group K (Table 4).
Ramsay sedation scale at baseline and at different time intervals after drug administration between groups. RSS: Ramsay sedation scale. Group D: dexmedetomidine group, Group K: ketamine group.
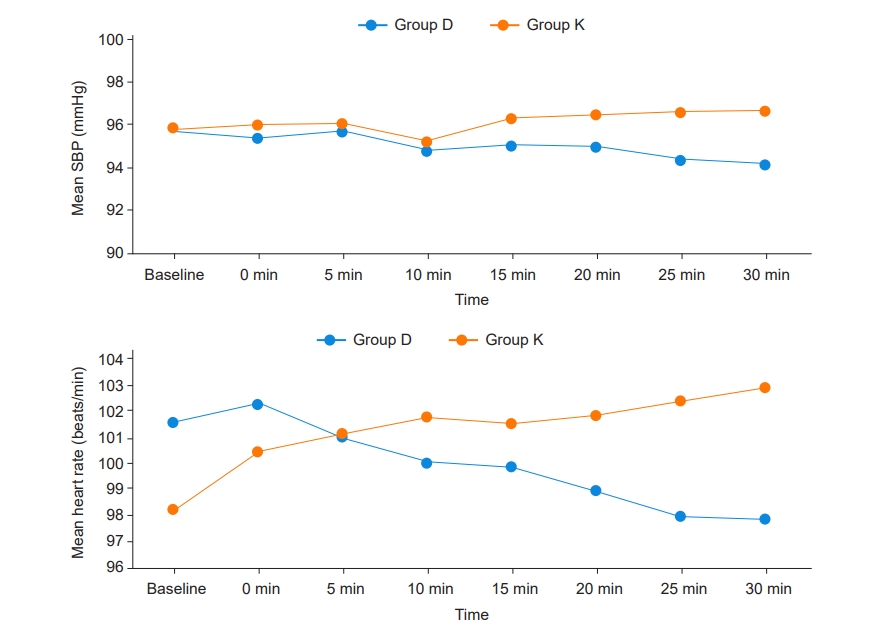
Baseline hemodynamic parameters were comparable between the groups. At 25 min and 30 min after premedication, there was a statistically significant difference in the mean HR (P = 0.028, 0.011) between the two groups. Repeated measures ANOVA showed a significant time effect in HR (Wilks’ Lambda = 0.395, P < 0.001) between groups (Fig. 3). At 25 min and 30 min after premedication, there was a statistically significant difference in mean SBP (P = 0.025, 0.015). Repeated measures ANOVA showed no significant time effect in SBP (Wilks’ Lambda = 0.844, P < 0.384) between groups.
Hemodynamic parameters (mean heart rate and mean systolic blood pressure) between groups. SBP: systolic blood pressure, HR: heart rate. Group D: dexmedetomidine group, Group K: ketamine group.
Intraoperative and postoperative hemodynamic parameters were comparable in both groups. None of the patients reported an episode of bradycardia or hypotension requiring intervention in either group after premedication. Thirty minutes after removal of LMA, 2.8% of children in the dexmedetomidine group and 8.5% of children in the ketamine group had EA. One patient in group D and two patients in group K complained of vomiting, and one patient in group K reported hypersalivation in the postoperative period.
DISCUSSION
The findings of this study were higher sedation scales achieved at 30 min, satisfactory parent child separation, and better mask acceptance in children premedicated with nebulized dexmedetomidine compared to nebulized ketamine. Moreover, both drugs had comparable hemodynamics and incidence of postoperative agitation.
Anxiolysis and smooth parental separation are important components of pediatric preoperative preparation. Various drugs, alone and in combination, via different routes were used. The nasal route of drug administration bypasses the enterohepatic circulation, which leads to better bioavailability and avoids the bitter taste of the drug compared to orally administered drugs. The atomizer used for nebulization creates small particulate forms of the drug, creating a thin layer around the buccal, nasal, and respiratory mucosa [9]. The inhaled drugs administered through nebulization were comparatively more effective, quicker onset, and safer than oral or intranasal routes for pediatric premedication [8,9,11,12].
Dexmedetomidine acts on α-2 adrenergic receptors of the locus coeruleus, resulting in quicker onset of sedation, mimicking natural sleep with less respiratory depression. Simultaneously, dexmedetomidine sedation is characterized by easy arousability without affecting the orientation and cooperation of the patient. The bioavailability of dexmedetomidine by non-intravenous routes, such as orogastric (16%), intranasal (65%), buccal (82%), and intramuscular (104%) [17–19].
Ketamine, a phencyclidine derivative, acts on the N-methyl-D-aspartate receptor and produces a state of dissociative anesthesia. Ketamine has been a popular drug for premedication in pediatric patients administered through various routes, but may result in excessive salivation, EA, or postoperative nausea and vomiting [20]. The pharmacokinetic properties of drugs administered through the nebulized route are still being investigated.
At 30 min, the majority of children (16/35, 45.7%) in the nebulized dexmedetomidine group had an acceptable sedation scale of 3, while only a few children (8/35, 22.8%) in the nebulized ketamine group had a sedation scale of 3. Similar results were found in a study by Sabry et al. [13], although they used higher doses compared to our study. They found comparatively calm, sedated children with better mask acceptance and fewer respiratory complications in the group receiving nebulized dexmedetomidine compared to the nebulized ketamine group and a mixture of nebulized ketamine and dexmedetomidine. This indicates that the dexmedetomidine group provided better sedation than the ketamine group.
Intranasal dexmedetomidine (2 µg/kg) was as effective as intranasal dexmedetomidine combined with ketamine (2 µg/kg + 1 mg/kg) for sedation during transthoracic echocardiography, with shorter recovery and discharge times, although the onset time was longer. The probable reason for delayed recovery and prolonged hospital stay in the combination group could be the excessive dose used for premedication [21].
However, both drugs have a different pharmacokinetic profile and their optimal dosage for combination to provide synergistic action while minimizing hemodynamic perturbation, and delayed recovery has not been established yet [22]. Due to delayed recovery with combination therapy, a single drug was used for nebulization in this study. A similar dosage of dexmedetomidine and ketamine via nebulization has been used in previous studies [11,12].
In this study, the PSAS was more satisfactory and MAS scores were higher in the dexmedetomidine group than in the ketamine group. These findings were in accordance with those of a previous study [11]. We also observed a decrease in HR and SBP from the baseline in the dexmedetomidine group due to the cardio-depressant effect, but slightly increased HR and SBP from the baseline in the ketamine group due to sympathetic stimulant properties of ketamine. A statistically significant difference was observed in HR and SBP at 30 min between the two groups. However, hemodynamic changes were clinically insignificant; hence, none of the patients required corrective interventions. Comparable hemodynamics in both groups reflect the usage of a smaller dosage and slower absorption of the drug from the nebulized route; hence, the plasma concentration for producing adverse effects was not built up in the body. Previous studies also demonstrated comparable hemodynamics with nebulized dexmedetomidine, ketamine, or a mixture of both as premedication in children [11–13].
The incidence of EA in pediatric patients ranges from 10 to 60%. In our study, EA was comparable between the groups. A recent systematic review and meta-analysis concluded that dexmedetomidine significantly decreased the incidence of post-anesthesia EA in pediatric patients compared with placebo, midazolam, and opioids [23]. Similarly, Ng et al. [24] also demonstrated the efficacy of ketamine in reducing EA in children undergoing surgery.
There are a few limitations to the present study. The serum concentrations of dexmedetomidine and ketamine after nebulization were not measured because of the lack of kits required for assays. The bioavailability of drugs varies according to the route of administration, and further studies are required to determine the pharmacokinetics and optimal dosage of these drugs administered through nebulization. Second, the time of onset of sedation was not compared in the present study. Third, the scoring system used for the determination of parental separation and mask acceptance was not validated. Lastly, the results of our study cannot be extrapolated for children younger than 2 years of age.
Dexmedetomidine nebulization had better, acceptable sedation along with smooth separation from parents and had satisfactory acceptance of face mask for induction of general anesthesia compared to nebulized ketamine. Moreover, they had a similar profile for emergence agitation and the incidence of drug-related adverse reactions.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Geeta Singariya, Namita Malhotra, Manoj Kamal, Rishabh Jaju, Pooja Bihani. Data curation: Namita Malhotra. Formal analysis: Namita Malhotra, Manoj Kamal, Shruti Aggarwal. Methodology: Geeta Singariya, Namita Malhotra, Manoj Kamal, Rishabh Jaju, Shruti Aggarwal, Pooja Bihani. Project administration: Geeta Singariya, Namita Malhotra, Manoj Kamal, Shruti Aggarwal, Pooja Bihani. Writing - original draft: Namita Malhotra, Shruti Aggarwal. Writing - review & editing: Geeta Singariya, Manoj Kamal, Rishabh Jaju, Shruti Aggarwal, Pooja Bihani. Investigation: Geeta Singariya, Namita Malhotra, Rishabh Jaju, Shruti Aggarwal, Pooja Bihani. Software: Rishabh Jaju. Supervision: Geeta Singariya, Pooja Bihani.