Choice of neuromuscular block reversal agent to reduce postoperative pulmonary complications
Article information
Abstract
The definition of postoperative pulmonary complications (PPCs) is inconsistent in literature; however, PPCs include pulmonary abnormalities that adversely affect patient outcomes, such as respiratory failure, atelectasis, pneumonia, pleural effusion, and exacerbation of underlying lung conditions. Furthermore, although the incidence of PPCs varies according to its definition, surgery type, and patient population, they can lead to increased morbidity, mortality, duration of hospitalization, and medical costs; thus, efforts to identify and reduce the risk factors are important to improve patient outcomes. Among the risk factors for PPCs, residual neuromuscular block is a representative and preventable anesthesia-related risk factor that is affected by the choice of the reversal agent. However, it is not clear whether the chosen reversal agent, i.e., sugammadex, reduces PPCs better when compared to anticholinesterases. Additionally, the effects of the reversal agents on PPCs in high-risk patients, such as elderly patients, pediatric patients, those with end-stage renal disease, obesity, obstructive sleep apnea, or those undergoing specific surgeries, are diverse. To reduce the PPCs associated with the use of neuromuscular blocking agents, it is important to confirm complete reversal of the neuromuscular block under neuromuscular monitoring. Additionally, efforts to reduce the incidence of PPCs through interdisciplinary communication are required.
INTRODUCTION
Postoperative pulmonary complications (PPCs) have been inconsistently defined in clinical trials, but they usually include pneumonia, atelectasis, respiratory failure, and reintubation [1]. The incidence of PPCs reportedly varies from less than 2% to 70%, depending on the type of surgery, surgical population, and definition of PPCs [2–6]. PPCs are the most important and independent determinants of 30-day mortality, and nearly 25% of the mdeaths occurring within 1 week after surgery are related to PPCs [3,7]. Additionally, because PPCs cause an increase in morbidity, medical costs, and duration of hospitalization, the identification of its risk factors and application of strategies to reduce PPCs are important clinically.
Many physicians struggle to reduce the risk of increased morbidity and mortality in patients undergoing surgery. Most factors that contribute to the occurrence of PPCs are related to surgery or the patients [8]. Moreover, as risk factors for PPCs, the strength of the evidence for anesthesia-related factors is weaker than that for surgery- or patient-related factors [3,8]. Nevertheless, strong evidence has shown that residual neuromuscular block (NMB) is associated with an increased risk of PPCs [1]. Therefore, it is important to understand the factors related to the field of anesthesia and make efforts to prevent them.
Among the anesthesia-related factors, the use of NMB agents (NMBAs) is known to be associated with increased PPCs as well as residual NMB, since the first report of its contribution to postoperative mortality in 1954. However, the relationship between PPCs and the type of reversal agents for NMB, such as conventional anticholinesterases and the relatively new sugammadex, remains debatable. This review discusses the important research findings on PPCs in the field of neuromuscular research.
POSTOPERATIVE PULMONARY COMPLICATIONS: DEFINITION, RISK FACTORS, AND PREDICTION MODELS
PPCs are not a new concept. They have existed since long and remain a subject of interest for all anesthesiologists, physicians, and surgeons involved in perioperative medicine [9–11]. Since there is no fixed definition for PPCs, various definitions have been used over time. The commonly accepted definitions of PPCs include the European Perioperative Clinical Outcome (EPCO) definitions published in 2015 and a new definition published in 2018 [12,13]. In the EPCO definitions, PPCs comprise respiratory infections, respiratory failure, pleural effusion, atelectasis, pneumothorax, and aspiration pneumonitis [12]. On the other hand, the new definition of PPCs announced in 2018 comprises atelectasis detected on computed tomography or chest radiography, pneumonia diagnosed according to the US Centers for Disease Control criteria, acute respiratory distress syndrome according to the Berlin consensus definition, and pulmonary aspiration with no clinical history or radiological evidence [13].
The causes of PPCs are multifactorial, and the related risk factors can be classified as preoperative or intraoperative. Preoperative factors include advanced age, American Society of Anesthesiologists physical status (ASA PS) ≥ II, frailty, chronic obstructive pulmonary disease, recent upper respiratory tract infection, obstructive sleep apnea (OSA), serum albumin level < 30 g/L, alcohol use, delirium, and abnormal chest radiography findings [1,8]. Intraoperative factors include surgical factors such as surgical site (e.g., abdominal, thoracic, neurosurgery, head and neck, or vascular), duration of surgery (> 2 h), and emergency surgery- or anesthesia-related factors such as general anesthesia or regional anesthesia, use of NMBAs, neostigmine administration, residual NMB, sugammadex with supraglottic airway, and no use of peripheral nerve stimulator [1,8,14].
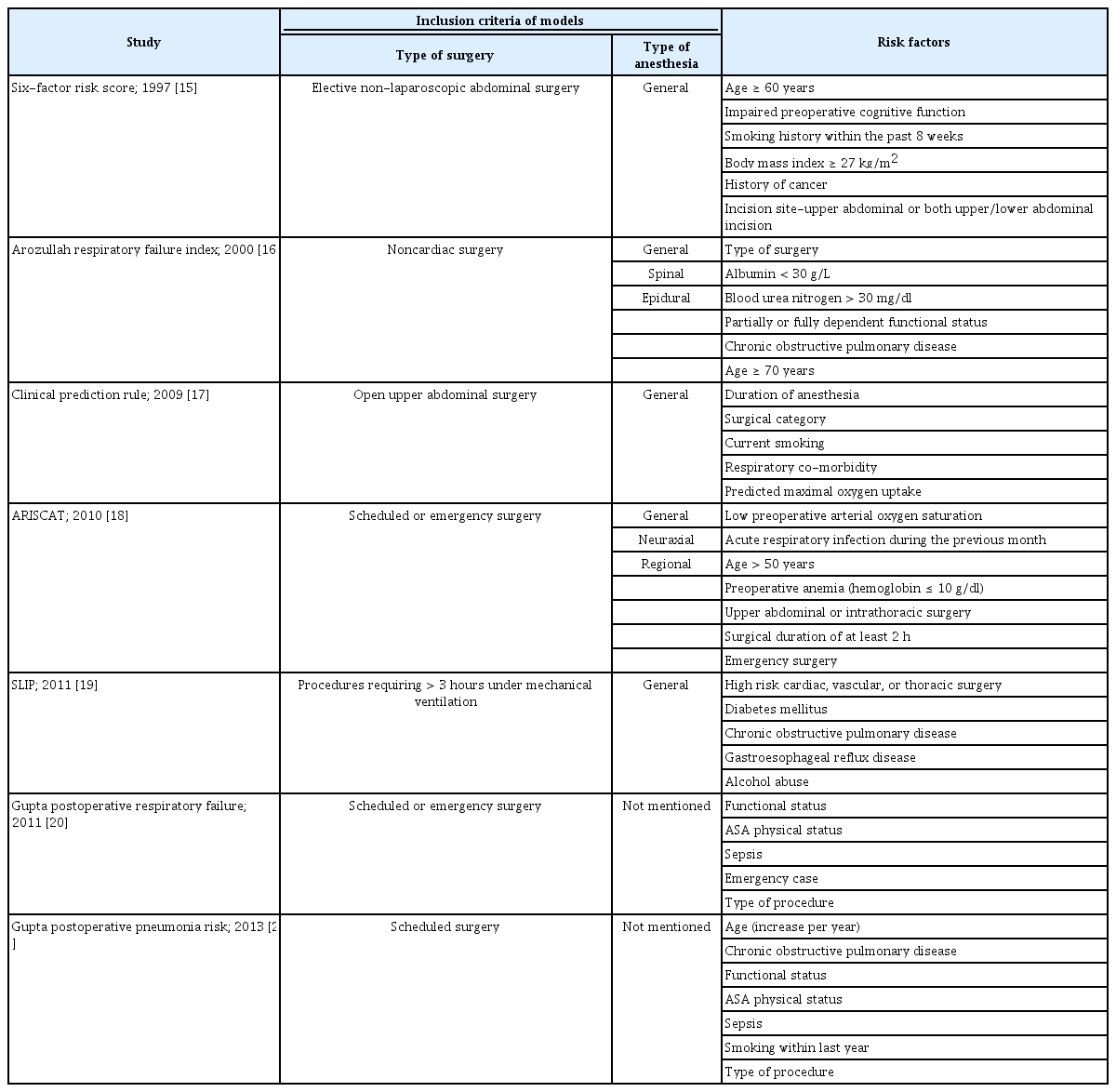
Several risk score models have been proposed to predict PPCs, but there is no “one-size-fits-all” model for the risk stratification for PPCs, and most of them have certain limitations [7,14]. To date, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score is the only predictive model that has shown sufficient predictive power in external validation [14]. Notably, only few models among the independent variables of several predictive models for PPCs, including the ARISCAT model, include anesthesia-related factors such as the use and reversal of NMBAs and anesthesia techniques (Table 1) [15–21]. This is probably because surgical factors, such as surgical site, and emergency surgery- and patient-related factors, such as age and underlying diseases, have a greater effect on PPCs than do the anesthetic factors [8,22]. Nevertheless, since the anesthesia-related factors, such as residual NMB, are modifiable and preventable unlike other factors such as age, surgical site, emergency surgery conditions, and surgical duration, understanding the effects of NMB and its reversal on PPCs is clinically relevant.
RESIDUAL NEUROMUSCULAR BLOCKADE AND POSTOPERATIVE PULMONARY COMPLICATIONS
Residual NMB, often defined by a train-of-four (TOF) ratio < 0.9, is one of the well-known anesthesia-related risk factors for PPCs. It is almost clear that residual NMB is associated with postoperative upper airway muscle dysfunction [23–25]. The main mechanisms of PPCs induced by residual NMB due to the NMBAs used during general anesthesia are respiratory muscle dysfunction, impairment of the hypoxic ventilatory response, and inability to protect the airway during swallowing. These pathological mechanisms may lead to adverse respiratory events such as atelectasis, hypoxia, aspiration, pneumonia, and reintubation [24].
Two representative strategies that anesthesiologists implement to avoid this potentially dangerous residual NMB include quantitative neuromuscular monitoring and appropriate reversal of the NMB. Neuromuscular monitoring in the perioperative period is essential to determine the degree of recovery from NMB through quantitative evaluation and to confirm the presence of residual NMB. A minimal level of NMB (TOF ratio, 0.7–0.9) cannot be detected without quantitative monitoring of the TOF [24,26]. To exclude clinically significant residual NMB, the TOF ratio must exceed 0.9, when recorded with mechanomyography or electromyography, and exceed 1.0 when using acceleromyography (AMG) [27].
Additionally, appropriate NMB reversal requires the administration of a titrated dose of the reversal agent according to the level of the block under quantitative neuromuscular monitoring [28]. Reversal agents for NMB are divided into the following two major categories: anticholinesterases (e.g., neostigmine and pyridostigmine) and sugammadex. Anticholinesterases bind to acetylcholinesterase, which can prolong the effects of acetylcholine and competitively antagonize NMBAs. However, it has a ceiling effect, with no effect above a certain dose, and NMB over a deep block cannot be reversed with anticholinesterases. In previous studies, proper administration of neostigmine under neuromuscular monitoring reduced the PPCs associated with the use of NMBAs; however, there were reports of inappropriate reversal using neostigmine, such as neostigmine administration not guided by the TOF ratio or administered dose > 60 µg/kg [29,30]. Thus, it should be used only when the twitch height is ≥ 20% of the control or when the minimum TOF count is two [31]. In contrast, sugammadex is a relatively new reversal agent approved in 2008 in Europe, 2012 in Korea, and 2015 in the United States, which can reverse the NMB at any stage by encapsulating only the aminosteroidal NMBAs [32]. Various studies have shown that sugammadex is associated with a lower incidence of residual NMB than that of neostigmine [33–35]. Hence, the question of whether PPCs can be reduced using sugammadex is raised.
REVERSAL AGENTS AND PPCS
Unlike anticholinesterases, which are traditional NMB reversal agents, sugammadex is the only reversal agent that can reverse deep and intense blocks. Moreover, various studies have confirmed that sugammadex can reduce the incidence of residual NMB [33–35]. Various meta-analyses performed for several randomized controlled studies comparing neostigmine and sugammadex reported that the incidence of respiratory events and residual curarization could be reduced with sugammadex [36–38]. However, it is difficult to use these studies as evidence that sugammadex reduces PPCs, because the PPCs in these studies were identified as one of the outcomes of various adverse events and not as the primary outcome. As an ideal condition of a randomized controlled trial (RCT) would minimize the overall incidence of PPCs and the difference between the two groups, the incidence of PPCs in the real world may be different from that in an RCT [39]. Therefore, it is important to investigate the relationship between reversal agents and PPCs through large-scale cohort studies.
A retrospective study published in 2014 with data from 1,444 patients reported that sugammadex could lower the risk of PPCs in high-risk patients with ASA PS ≥ III; however, this finding should be interpreted cautiously because the endpoints are mixed, including acute post-anesthetic care unit (PACU) complications, bronchospasm, airway intervention, cardiac arrhythmia, length of stay in the PACU, and pulmonary outcome within 7 days postoperatively [40]. The post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR) study published in 2019, supported by the European Society of Anaesthesiology and Intensive Care, is the first multicenter cohort study providing prospective data for PPCs and analyzing 22,803 patients to identify PPCs related to NMBAs [41]. The incidence of PPCs in that study was 7.8%. Moreover, it showed that the use of NMBAs was associated with an increase in PPCs, although the use of NMBA had a lesser effect on the risk of PPCs than did the type and duration of surgery or the patient’s preoperative pulmonary function. In contrast, monitoring of NMB, use of reversal agents, extubation at TOF ratio ≥ 0.9, and the use of sugammadex instead of neostigmine were not associated with a reduction in PPCs. Some of these results have been refuted in later studies [42,43]; PPCs were reduced with TOF ratio > 0.95 before tracheal extubation than with TOF ratio > 0.9 [42], and the use of sugammadex instead of neostigmine was associated with a decrease in PPCs [43]. In an exploratory analysis of the POPULAR data, applying a TOF ratio threshold of 0.95 before extubation instead of 0.9 showed a decrease in PPCs [42]. This is presumed to be due to the property of the AMG, which overestimates neuromuscular recovery. In the POPULAR study, 87% of the neuromuscular monitoring devices used were AMG, which requires “normalization” of the TOF ratio to obtain an accurate TOF ratio of 0.9. However, normalization is rarely performed in clinical practice. Additionally, NMB reversal was achieved only in less than half of the patients who received NMBAs, and the number of patients receiving sugammadex was < 2,000. Furthermore, only 20% of the patients had complete data that allowed for the matching of patients and comparison of the treatment methods. Therefore, a more appropriate conclusion might be that NMBAs can cause serious outcomes if managed improperly.
Until the sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER) study was published in 2020, no large-scale multicenter study had focused on the relationship between reversal agents and PPCs [43]. Although this study has limitations in collecting data retrospectively, its hypothesis was that patients receiving sugammadex have fewer PPCs than those receiving neostigmine. PPCs were confirmed in 4.1% of the 45,712 patients. The incidence of PPCs was 3.5% in the sugammadex group and 4.8% in the neostigmine group, confirming that the use of sugammadex reduced the risk by 30%. Moreover, and pneumonia and respiratory failure were reduced by 47% and 55%, respectively. However, since the data collection period of 5 years was set retrospectively, and the definition of PPCs was established using the prescription codes of the International Classification of Diseases-9 and -10, the results should be interpreted considering these limitations compared to prospective studies.
However, unlike previous studies [40,43], in a retrospective registry study on the occurrence of PPCs related to the use of sugammadex and neostigmine published in 2021 [44], there was no significant difference in the incidence of PPCs between them. In this study, the absolute incidence of PPCs decreased over time (adjusted odds ratio, 0.91 [per year]), which may be a simple change due to the long data collection period (10 years). In addition to the difference between the use of neostigmine and sugammadex, because of the multiple quality improvement initiatives over time including enhanced recovery after surgery protocols, the use of objective criteria for ventilator-related pneumonia and of TOF monitoring were implemented in the hospital where this study was conducted. All these factors may have influenced the reduction of PPCs.
Among the studies published so far on the occurrence of PPCs with sugammadex and neostigmine, a recently published meta-analysis analyzed 14 RCTs and 1,478 patients [45]. In the main meta-analysis, the risk of overall PPCs was lower with sugammadex than with neostigmine. In the stratified subgroup analyses, sugammadex was associated with a reduced risk of respiratory failure as compared to neostigmine, but there was no statistical difference in the occurrence of pulmonary infection, atelectasis, or pneumothorax. This meta-analysis may have clinical heterogeneity due to differences in patient comorbidities and the type of surgery. Additionally, PPCs such as respiratory infections, atelectasis, and pneumothorax were included only in 1–3 studies. These limitations should also be clearly considered [45].
Despite the discussions in recent studies, it is difficult to determine the exact association between the choice of reversal agent and PPCs. Published retrospective studies were unable to control for several factors that affect PPCs. However, controlled situations do not accurately reflect the actual clinical environment. In a clinical environment, the individual patient characteristics and the condition according to the risk factors should be understood and considered.
RECENT RESEARCHES IN SPECIFIC PATIENT POPULATIONS OR SURGERIES
As mentioned earlier, there are limited studies on the relationship between reversal agents and PPCs, and studies on certain specific populations and situations are lacking (Table 2).

Details of Studies Investigating the Relationship between the Reversal Agents for Neuromuscular Blockade and Postoperative Pulmonary Complications for Specific Patient Populations and Surgeries
Elderly patients
Old age is a well-known risk factor for PPCs, and two studies were recently published on PPCs following NMB reversal in the elderly [46,47]. In one RCT published in 2020, elderly patients aged > 70 years with ASA PS I–IV and scheduled for surgeries lasting > 3 h were divided into two groups [46]. Although the decrease in PPCs was not significantly different between sugammadex and neostigmine, the residual NMB and 30-day readmission rates were lower with sugammadex than with neostigmine. The other study published in 2021 was conducted in five countries, including South Korea, and it was confirmed that the occurrence of postoperative pneumonia and residual NMB was significantly lesser with sugammadex than with neostigmine in high-risk patients aged ≥ 75 years [47]. The difference between the two studies could be attributed to differences in the patient characteristics included in each study. Participants of the latter study (2021) included only those with ASA PS ≥ III and those aged ≥ 75 years, thus representing high-risk patients. Furthermore, it supported another study showing that PPCs in high-risk patients could be reduced with sugammadex used for NMB reversal [40].
Pediatric patients
Owing to the several restrictions on the use of sugammadex in children, as its pediatric use was not approved in South Korea until October 2021 and has been not yet approved in the United States for children, studies directly related to PPCs in children are rare. However, it has been found that sugammadex can be used safely and efficiently to reverse rocuronium-induced NMB in pediatric patients. Furthermore, studies showed no difference between sugammadex and neostigmine, except for the lower incidence of bradycardia with the former than with the latter [48,49]. In addition, a recent study confirmed that incidence of postoperative atelectasis, duration of hospitalization, and hospitalization costs were reduced when sugammadex was used in children undergoing congenital heart surgery [50]. In another study, postoperative C-reactive protein and procalcitonin levels were significantly lower with sugammadex than with neostigmine after cardiac surgery. It was expected that this would reduce the degree of lung inflammation and that extubation after using sugammadex could reduce complications caused by long-term ventilator use [51]. Even though sugammadex could be useful in children who need fast-track or early extubation after cardiac surgery, further research with PPCs as the primary outcome in pediatric patients is warranted.
Renal impairment
One of the concerns when using sugammadex in patients with end-stage renal disease (ESRD) is that the sugammadex-rocuronium complex or free sugammadex may cause recurarization or anaphylactic reactions because the clearance of sugammadex is reduced in these patients [52–56]. Although studies have been conducted on the usefulness of sugammadex in ESRD patients, only few have confirmed the use of sugammadex to reduce respiratory complications [57–59]. The two studies published in 2020 were retrospective studies that could only confirm incidence of PPCs, but the definition was not suggested [60,61]. The first study investigated hypersensitivity or respiratory complications that could occur because of delayed sugammadex excretion in ESRD patients [60]. All complications reported in that study were respiratory complications without hypersensitivity, and 25 instances of complications were reported in 18 out of 219 patients. The second study retrospectively analyzed 158 ESRD patients out of 26,650 patients receiving sugammadex, and 3 of 136 patients who were extubated in the operating room needed mechanical ventilation within 48 h. Of these cases, two were caused by pulmonary edema due to volume overload and one by sepsis [61]. None of them were due to the recurrence of NMB. Although some studies have investigated the efficacy and safety of sugammadex use in ESRD patients [61,62], evidence regarding the effect on PPCs in ESRD patients is insufficient, and more data and research are required.
Obesity
Obesity causes various anatomical and physiological changes in the human body. This not only includes the anatomical changes caused by fat accumulation, but also physiologic changes in respiration, such as excessive tissue metabolic requirements, increase in respiratory workload, increased oxygen consumption and carbon dioxide production, and ventilation/perfusion mismatching, making the patients vulnerable to hypoxia and apnea [63–66]. These changes could be one of the factors increasing the incidence of respiratory complications postoperatively. However, the evidence and research showing that sugammadex reduces PPCs in patients with obesity or OSA are lacking. Therefore, the studies on obese patients mentioned below included the results of OSA patients representing obese patients. According to a systematic review published in 2018, it was expected that the use of sugammadex could reduce PPCs when compared to the use of neostigmine in patients with OSA, but the relevant evidence was limited [67]. Among the two studies analyzed in this systematic review, a prospective observational study of patients undergoing laparoscopic bariatric surgery showed that the requirement of mechanical ventilation did not differ significantly between sugammadex and neostigmine (1.25% vs. 3.1%) [68]. However, postoperative pathological findings, including atelectasis or pleural effusion on chest radiography, were significantly lesser with sugammadex than with neostigmine. Another study on patients with OSA in 2015 showed that desaturation, reintubation, and unplanned intensive care unit admissions were significantly lower with sugammadex than with neostigmine [69]. However, in both these studies, the primary outcome was not PPCs, the definition of PPCs was not clear, and it is uncertain whether the results are clinically relevant. Therefore, this target group requires further study.
Thoracic and abdominal surgery
The surgical site is the most important risk factor for PPCs [8]. PPCs are different from cardiac complications; even in healthy adult patients, the risk of PPCs is high if the surgical site is intrathoracic or in the upper abdomen [8,18]. Intrathoracic surgery is associated with increased incidence of atelectasis and other PPCs because of the need for one-lung ventilation during surgery [5]. In a retrospective observational study of patients undergoing open lung lobectomy, the primary outcomes of hospitalization duration and postoperative atelectasis were significantly lower with sugammadex than with pyridostigmine [70]. Similarly, in a retrospective study of patients undergoing single-port video-assisted lung lobectomy, early postoperative abnormalities on chest radiography were significantly lesser with sugammadex than with pyridostigmine [71]. However, according to an RCT conducted on patients undergoing video-assisted lung lobectomy in 2021, there was no difference in the incidence of PPCs between sugammadex and neostigmine [72]. The extubation criterion was set at TOF ratio ≥ 0.9 in the RCT study, suggesting the possibility that the complications were not different. Therefore, it was confirmed that PPCs may not simply differ depending on the choice of the reversal agent, and it is important to reverse the NMB completely. Additionally, abdominal surgery is one of the major factors increasing the risk of PPCs [1,8,14]. According to a retrospective study investigating PPCs in patients undergoing laparoscopic gastrectomy, the incidence of pleural effusion was significantly reduced with sugammadex, and the incidence of other PPCs (respiratory failure, pneumonia, aspiration pneumonitis, atelectasis, and pneumothorax) did not differ between sugammadex and neostigmine [73]. In a retrospective observational study that compared sugammadex and neostigmine with the 30-day readmission rate as the primary outcome in patients undergoing major abdominal surgery, the use of sugammadex reduced the 30-day readmission rate by 34% [74]. However, in an RCT comparing sugammadex and neostigmine, sugammadex did not significantly improve the change in the forced vital capacity, which was the primary outcome, nor did it reduce the incidence of atelectasis after major abdominal surgery [75]. Therefore, the effect of sugammadex on PPCs during abdominal surgery remains debatable.
CONCLUSION
Although several efforts are being made to reduce PPCs, it remains questionable whether the choice of the NMB reversal agent affects PPCs. To date, complete reversal of the NMB before extubation under neuromuscular function monitoring seems more important than choosing a reversal agent. Moreover, understanding and considering the characteristics of each patient and the surgery type are important to reduce PPCs. As mentioned in the beginning, because the strength of evidence regarding surgical or patient-related factors as risk factors for PPCs is greater than that for anesthesia-related factors, a multidisciplinary approach should be considered to reduce PPCs.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
AUTHOR CONTRIBUTIONS
Conceptualization: Tae-Yun Sung. Writing - original draft: Sung-Ae Cho, Tae-Yun Sung. Writing - review & editing: Sung-Ae Cho, Tae-Yun Sung.