Hyperekplexia with congenital heart disease: anesthetic concerns and management
Article information
TO THE EDITOR: Hyperekplexia, also known as startle disease, is a rare genetic neurological disorder that primarily affects infants and is characterized by excessive blinking of eyes or body spasms in response to sudden unexpected auditory or tactile stimulation. Symptoms include extreme muscle tension (stiffness or hypertonia) [1]. Hyperekplexia is usually inherited as an autosomal dominant trait, but autosomal recessive or, rarely, X-linked inheritance may also occur [2]. Mutations in GLRA1, SLC6A5, GLRB, GPHN, and ARHGEF9 (X-linked) have been associated with these conditions. Hyperekplexia 1 is caused by a mutation in GLRA 1 gene [3]. Delayed developmental milestones or learning difficulties can be observed in some children. Congenital disabilities leading to dysfunction of glycinergic inhibitory transmission are seen in hereditary hyperekplexia [4,5]. They are frequently associated with inguinal, umbilical, or epigastric hernias. As a rare disease, limited literature is available regarding anesthetic management in such cases, primarily when associated with congenital heart disease (CHD). Here, we discuss the successful management of a hyperekplexic child with CHD.
Written informed consent to publish this case was obtained from the patient's legal guardian.
A 1-year-old child weighing 9 kg was scheduled for a right inguinal hernia repair surgery. At presentation, the patient had dysmorphic facial features (short nose, retrognathia, anteverted nares, and long philtrum) (Fig. 1). His medical history suggested that he was born by caesarean section (because of thick meconium-stained liquor). The patient’s birth history was uneventful. However, a few hours after birth, he presented with a flexed posture, brisk reflexes, and an exaggerated startle response and was diagnosed with hyperekplexia. At one month of age, he developed global hypertonia, and had delayed development. Electroencephalography revealed interictal epileptiform discharges originating from the left posterior head region. Electromyography revealed a myopathic potential. Magnetic resonance imaging results were normal. Echocardiography showed mild pulmonary stenosis and ballooning of the pulmonary valve (pulmonary valve area 2.06 square meter and pulmonary artery systolic pressure, 17 mmHg) with a small atrial septal defect. All other investigations were normal except hemoglobin, which was 8.6 g/dl. The child was on tablet diazepam (2.5 mg) twice daily and syrup baclofen (5 mg/5 ml) 1.5 ml twice daily. These medications were continued in the perioperative period.
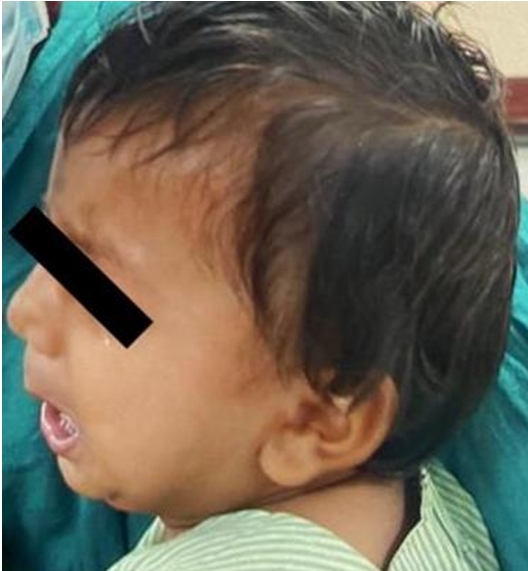
The dysmorphic facial features (short nose, retrognathia, anteverted nares, long philtrum) of the child.
The child was premedicated with midazolam (0.05 mg/kg), ketamine (0.5 mg/kg), and glycopyrrolate (0.005 mg/kg). Induction was performed with fentanyl (2 μg/kg) and propofol (2 mg/kg) at titrated doses. I-gel no. 1.5 was inserted. Muscle relaxants were avoided. Anesthesia was maintained with oxygen in air (50:50), and isoflurane at a minimum alveolar concentration (MAC) of 1.0–1.2. A caudal block was administered with 9 ml of 0.2% ropivacaine to provide intraoperative and postoperative analgesia. The patient’s intraoperative course was uneventful.
In our case, we preferred propofol over etomidate as an induction agent because propofol possesses antiepileptic properties and rarely causes myoclonic jerk. This contrasts with etomidate, which frequently produces myoclonic jerk. Thus, propofol is a better choice for treating children with hyperekplexia. Although propofol decreased systemic vascular resistance compared to etomidate, pulmonary stenosis in our case was mild. If severe pulmonary stenosis is present, etomidate (a stable cardiovascular drug) may be a better choice. In our case, we used ketamine to provide sedation and analgesia, thus preventing separation anxiety. It also prevents a decrease in the systemic vascular resistance.
We preferred a supraglottic airway device (I-gel, Inter-surgical Ltd., UK) for tracheal intubation. The patient had dysmorphic facial features, indicating difficult tracheal intubation, thereby, avoiding multiple intubation attempts and hemodynamic responses to laryngoscopy. Anesthesia was maintained with isoflurane traces by achieving and maintaining a MAC value of 1.0–1.2 without a muscle relaxant, thus avoiding untoward muscle relaxant effects in the patient. There is no definite anesthetic plan for conducting a case of hyperekplexia. To date, little is known about the correlation between GLRA1 and muscle relaxants. There is a vast scope for research on this disease. We conducted this case by maintaining an adequate depth of anesthesia with analgesia, and without a muscle relaxant.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: Manbir Kaur. Data curation: Manbir Kaur, Raksha Vyas. Formal analysis: Raksha Vyas, Pradeep Bhatia. Visualization: Raksha Vyas, Tanvi Meshram. Writing - original draft: Manbir Kaur. Writing - review & editing: Tanvi Meshram, Pradeep Bhatia. Supervision: Tanvi Meshram, Pradeep Bhatia.
