 |
 |
- Search
| Anesth Pain Med > Volume 18(2); 2023 > Article |
|
Abstract
Background
Perioperative hyperglycemia can occur in surgical patients and may increase postoperative morbidity and mortality, especially in patients with diabetes. Therefore, we conducted the present study to evaluate whether the administration of 6% hydroxyethyl starch (HES)-130/0.4 increases blood glucose levels in patients with diabetes.
Methods
Forty patients undergoing lower limb surgery under spinal anesthesia were randomly allocated into two groups according to the fluids administered 20 min before spinal anesthesia (Group L, lactated Ringer’s solution; Group H, 6% HES-130/0.4). Patient characteristics, intraoperative variables, blood glucose levels, mean blood pressure (MBP), and heart rate (HR) were recorded at five time-points (0, 20, 60, 120, and 240 min).
Results
A total of 39 patients were analyzed (Group L, n = 20; Group H, n = 19). The amount of intraoperative fluid was significantly higher in Group L than in Group H (718.2 ml vs. 530.0 ml, P = 0.010). There were no significant differences in the changes in blood glucose levels, HR, or MBP between the two groups (P = 0.737, P = 0.896, and P = 0.141, respectively). Serial changes in mean blood glucose levels from baseline also showed no significant differences between the groups (P = 0.764).
Conclusions
There were no significant changes in blood glucose levels when lactated Ringer’s solution or 6% HES-130 was used. When compared to the lactated Ringer’s solution, no evidence that 6% HES-130/0.4 produces hyperglycemia in diabetic patients could be found. Further evaluation of larger populations is needed.
Surgical patients often show high blood glucose levels, which are known to be caused by a hypermetabolic response to various surgical or anesthetic stresses [1,2]. The catabolic nature of human homeostasis results in the activation of endocrine systems, such as the hypothalamic-pituitary-adrenocortical pathway, which leads to changes in the regulation of hormones [1-3]. Increased secretion of counterregulatory hormones, such as catecholamines, cortisol, glucagon, or growth hormone, promotes gluconeogenesis and hepatic glucose production, all while inhibiting insulin release by pancreatic β-cells [1]. These changes in hormone levels and metabolism lead to increased blood glucose levels and interrupt glucose control during surgery.
Perioperative hyperglycemia is significantly related to adverse clinical outcomes and is thought to be an independent risk factor for postoperative morbidity and mortality [1,4,5]. Perioperative hyperglycemia can occur regardless of whether a patient has diabetes, and its incidence in patients undergoing general surgery ranges between 20 and 40% [1]. However, patients with diabetes may require more frequent surgical interventions and hospitalizations [6]. Moreover, diabetic patients can have worse surgical outcomes, including acute diabetic complications, as well as show increased infection rates, delayed wound healing, longer hospital stays, and worse perioperative mortality rates than non-diabetic patients [6]. Therefore, diabetic patients undergoing surgery should be carefully considered to avoid the risk of hyperglycemia during surgical procedures [4].
Although there are concerns regarding side effects in some clinical situations [7], hydroxyethyl starch (HES), a synthetic carbohydrate polymer, is still used for fluid resuscitation, as colloids still have advantages for intravascular volume expansion in clinical hypovolemia [7-9]. HES can also be used to prevent hypotension after spinal anesthesia [10]. HES is a derivative of amylopectin and may have the potential to increase blood glucose levels, as amylopectin is a highly branched compound of starch that resembles glycogen in structure, is rapidly hydrolyzed by amylase, and has a half-life of approximately 20 min [7,11]. Several studies have reported that 6% HES-130/0.4 does not increase blood glucose levels or cause slight increases within physiological limits in surgical patients without diabetes [11-13]. However, there are objections that HES (6% HES-450 or 6% Pentastarch-200) significantly increases blood glucose levels [14,15]. Moreover, controversy remains regarding whether 6% HES-130/0.42 causes a significant increase in blood glucose levels in surgical patients with diabetes [16].
In consideration of the above, we conducted the present study to evaluate whether preloading of 6% HES-130/0.4 increases blood glucose levels compared to control lactated Ringer’s solution in patients with diabetes undergoing lower limb surgery under spinal anesthesia.
After approval from Institutional Review Board of Chosun University Hospital (2014-10-003), we carefully explained the study and obtained written informed consent from the patients. We conducted this randomized, double-blind, controlled study in accordance with the Ethical Principles for Medical Research Involving Human Subjects outlined in the Helsinki Declaration of 1975 (revised 2013).
A total of 40 patients with type 2 diabetes mellitus who were undergoing spinal anesthesia for elective surgery of the lower limb (American Society of Anesthesiologists physical status classification II and aged between 30 and 80 years) in our hospital were enrolled in the study. The inclusion criteria were as follows: body weight within 40 to 75 kg, taking only oral medications for glucose control, and well-controlled glucose levels that did not require hospitalization (hemoglobin A1c, HbA1c < 7.0%) [12,14]. The exclusion criteria were as follows: uncontrolled diabetes; diabetes patients treated with insulin; taking of drugs such as acetaminophen, steroids, or ascorbic acid, which can cause hyperglycemia continuously; history of allergy to corn or other experimental drugs; dysfunction of the kidney, liver, or heart; coagulation disorders; and hypovolemia or hypervolemia, including pulmonary edema.
All recruited patients agreed to participate in the study and were allocated in a 1:1 ratio according to computer-generated random numbers. An independent anesthesiologist prepared the sealed envelopes, and patients were allocated into two groups according to the number of sealed envelopes in sequential order. Group L (n = 20) was administered lactated Ringer’s solution (15 ml/kg; JW Pharmaceutical, Korea). Group H (n = 20) was administered 6% HES 130/0.4 (Volulyte; Fresenius Kabi; 7.5 ml/kg).
Diabetes was managed following the guidelines of the consultant to the Department of Medicine [17,18]. On the morning of surgery, the patients were advised not to take any oral diabetes medications. After overnight fasting, blood glucose levels were measured in the ward. Hyperglycemia with blood glucose > 200 mg/dl was treated with small doses of short-acting insulin (0.1 U/kg). Hypoglycemia < 70 mg/dl was treated with dextrose 50% solution. Eventually, the patients’ blood glucose levels were maintained within the target range (80-180 mg/dl) before surgery [19]. Any patient with poor glycemic control before the surgery dropped out of the study. During the study, the patients fasted.
After obtaining intravenous access with an 18-gauge intravenous cannula and following the administration of intramuscular midazolam (0.05 mg/kg), patients were transferred to the waiting room in the operating room (OR). Basal blood glucose levels (baseline, T0) were measured using a glucometer (Accu-Check Inform II METER, Roche Diagnostics GmbH) using blood samples from participants’ fingertips. For blinding, an independent nurse administered the experimental fluids 20 min before the induction of anesthesia in the waiting room. The patients were transferred to the OR after changing the fluids to physiological saline (JW Pharmaceutical), which was maintained until the end of the study. Physiological saline was administered freely during surgery to maintain blood pressure within 30% of the baseline. Upon arrival at the OR, basal monitoring was performed using electrocardiography, pulse oximetry, and non-invasive arterial pressure. An anesthesiologist blinded to the patient allocation conducted the spinal anesthesia. After lateral decubitus positioning of the patients, spinal anesthesia was performed at the L4-5 intervertebral space using a 25-G Quincke needle. Hyperbaric bupivacaine hydrochloride 0.5% (Marcaine Spinal Heavy®, Astra Zeneca) without an adjuvant was administered intrathecally. Non-invasive blood pressure was monitored at 5-min intervals. A bolus of 50 μg of phenylephrine (Hana Pharm) was injected when systolic pressure decreased by more than 80% of the baseline or was below 90 mmHg.
The primary outcome of this study was to compare changes in blood glucose levels according to the preloading of the designated fluids. Blood glucose levels were measured by blinded anesthesiologists and nurses according to the following time intervals: T0, baseline before administration of the designated fluid; T1, 30 min after administration of the designated fluid; T2, 1 h after administration of the designated fluid; T3, 2 h after administration of the designated fluid; and T4, 3 h after administration of the designated fluid. The means and differences from the baseline blood glucose levels were calculated and compared at each time point. Secondary outcomes such as mean blood pressure (MBP) and heart rate (HR) were measured simultaneously when blood glucose levels were assessed. Patient characteristics and perioperative variables such as age, sex, height, weight, body mass index (BMI), comorbidity, preoperative HbA1c, blood glucose level on the morning of surgery, fasting time, type and duration of surgery, duration of tourniquet use, amount of administered bupivacaine, block level, number of patients requiring phenylephrine use, total administered dose of phenylephrine, amount of intraoperative fluid, and estimated blood loss were recorded.
The sample size was calculated using G*Power3 free software (Franz Faul, University of Kiel, Germany). First, the effect size was calculated based on a previous study that compared blood glucose levels after administration of lactated Ringer’s solution and 6% HES [12]. However, according to the data from a previous study (effect size: 1.75, variance explained by special effects: 8.54, variance within groups: 9), the calculated sample size was too small [20]. Therefore, we assumed the effect size to be 0.3 according to Cohen’s guidelines for social science [21]. Based on the results of a previous study that compared blood glucose levels between baseline and 1 h after administration [12], the correlation among the repeated measures was calculated to be 0.25. The total sample size was 38 for the repeated-measures two-way ANOVA test; this was performed with five consecutive measurements of blood glucose levels, with α = 0.05, and with a power of 80%. A dropout rate of 5% was considered, and 20 patients were allocated to each group.
Statistical analyses were performed using SPSS software (version 21.0, IBM Co.). Normality tests were performed using the Shapiro-Wilk test. Normally distributed data (age, height, weight, BMI, preoperative HbA1c, blood glucose level on the morning of surgery, fasting time, duration of surgery, duration of tourniquet use, and amount of intraoperative fluid) were analyzed using Student’s t-test. Non-normally distributed data, such as the administered dose of bupivacaine, total administered dose of phenylephrine, and estimated blood loss, were analyzed using the Mann-Whitney U test. Categorical variables were analyzed using the Chi-square test (sex and number of patients requiring the use of phenylephrine) or Fisher’s exact test (comorbidity, type of surgery, and block level). Values were expressed as mean ± SD, median (1Q, 3Q), or number of patients (%) with exact P values. A repeated measures two-way ANOVA was performed to compare the differences in MBP, HR, and blood glucose levels between the groups. The changes in blood glucose levels according to the time sequence were also analyzed using repeated-measures two-way ANOVA. A Greenhouse-Geisser correction was applied to the data, as it did not satisfy the sphericity assumption after Mauchly’s test of sphericity. Post-hoc tests were performed using the Mann-Whitney U test. After Bonferroni correction, adjusted P values < 0.05 were considered statistically significant.
Forty patients were assessed for eligibility and enrolled. Patients’ blood glucose levels were maintained within the target range (80-180 mg/dl) on the morning of surgery and did not require further management with insulin or dextrose solution administration. One patient was excluded owing to the need to convert from spinal anesthesia to general anesthesia due to inadequate blockade. Finally, data from 39 patients (Group L, n = 20; Group H, n = 19) were analyzed (Fig. 1).
There were no significant differences in the patient characteristics or preoperative variables between the groups (Table 1). The perioperative outcomes also showed no significant differences, except for the amount of intraoperative fluid. The amount of intraoperative fluid was significantly higher in Group L than in Group H (718.2 ml vs. 530.0 ml, P = 0.011, Table 2).
The changes in mean blood glucose levels according to the time sequence did not show significant differences between the two groups (P = 0.737 with Greenhouse-Geisser correction). There were also no significant differences in the mean blood glucose levels at any of the five time points (Fig. 2). Serial changes in the mean blood glucose levels from the baseline values also showed no significant differences between the groups (P = 0.764 with Greenhouse-Geisser correction, Fig. 2).
Changes in MBP and HR did not show any significant differences between the groups (MBP, P = 0.896 with Greenhouse-Geisser correction; HR, P = 0.141). There were also no significant differences in MBP and HR at any of the five time points (Fig. 3).
In this study, the preloading of 6% HES-130/0.4 before spinal anesthesia showed no significant increase in blood glucose levels compared to the control lactated Ringer’s solution during lower limb surgery in patients with diabetes. Therefore, compared to lactated Ringer’s solution, we could not find evidence that 6% HES-130/0.4 produces hyperglycemia in diabetic patients.
The molar substitution of hydroxyl residues into hydroxyethyl residues of HES increases water solubility and delays amylase hydrolysis by amylase [7,9]. The average number of hydroxyethyl residues of 6% HES-130/0.4 is 4/10 glucose molecules [7]. Therefore, 6% HES-130/0.4 has six parts that have not been substituted for hydroxyethyl residues, which are easily hydrolyzed by amylase to raise blood glucose levels [7]. Previous studies have shown significant increases in blood glucose after using HES (6% HES-450 or 6% Pentastarch-200/0.5) in non-diabetic surgical patients [14,15]. However, other reports that studied changes in blood glucose levels after using 6% HES-130/0.4 showed no significant increase or a slight increase within physiological limits in non-diabetic surgical patients [11-13]. This difference is thought to be caused by the difference in the number of hydroxyethyl residue substitutions and the C2/C6 ratio according to the type of HES, which could affect the hydrolysis rate of HES by amylase [7,9]. In particular, the attachment of hydroxyethyl residues to the C2 molecules of glucose molecules strongly inhibits the hydrolysis of starch by amylase [7,9]. Therefore, HES with a higher C2/C6 ratio is hydrolyzed more slowly. Notably, the C2/C6 ratio of 6% HES-450 or 6% Pentastarch-200 is 5:1, whereas the C2/C6 ratio of 6% HES-130/0.4 is 9:1 [7].
Patients with diabetes who are undergoing surgery might be more susceptible to surgical stress, which changes the regulation of metabolism and hormones, leading to hyperglycemia easily [1-4]. Therefore, careful consideration of the selection of perioperative fluids is required for patients with diabetes. Recently, one study showed that preloading of 6% HES-130/0.4 30 min prior to general anesthesia in diabetic patients significantly increased blood glucose levels within the first hour of initial administration [16]. Moreover, the increase in blood glucose levels after preloading with 6% HES-130/0.4 was much greater in patients with diabetes than in those without diabetes [16]. However, our study showed no significant changes in the blood glucose levels of diabetic patients over 3 h, regardless of the type of fluid administered. It is thought that this discrepancy resulted from differences in the anesthetic methods, as this study employed spinal anesthesia instead of general anesthesia. A previous study comparing perioperative blood glucose levels in patients undergoing hip arthroplasty showed a significant increase in glucose levels in patients undergoing general anesthesia versus spinal anesthesia [22]. The glucose levels of the patients who received spinal anesthesia remained stable and required no additional glycemic control during surgery by attenuating the hyperglycemic response to surgical stimuli.
In addition to the above results, the blood glucose levels of the patients in the current study remained stable despite the use of HES, and the patients also did not require any glycemic control during the observational period. The following hypothesis can be considered as the cause of these results: First, the characteristics of 6% HES-130/0.4, including its high molecular weight, molar substitution, and high C2/C6 ratio, inhibit hydrolysis by amylase, which is likely the primary mechanism for the conversion of HES into glucose [7]. Second, degraded molecules from HES are small enough (molecular weight of approximately 40-50 kDa) to be excreted in the urine rapidly before amylase-dependent breakdown occurs [23]. Third, HES metabolism requires amylase and increases serum amylase to levels as high as twice the basal value [24]. However, serum amylase activity is significantly decreased in patients with type 2 diabetes mellitus [25]. Therefore, the conversion of HES to glucose may be delayed. Additionally, in the patients in this study, preloading of 6% HES-130/0.4 decreased the requirements for intraoperative fluids compared to lactated Ringer’s solution (718.2 ml vs. 530.0 ml, P = 0.011). In addition, as there were no significant differences in the administered doses of bupivacaine, block levels after spinal anesthesia, use of phenylephrine, or vital signs such as MBP and HR, the amounts of loading fluids after spinal anesthesia for the maintenance of vital signs were reduced in patients administered 6% HES-130/0.4 compared to those administered lactated Ringer’s solution.
The general incidence of spinal anesthesia-induced hypotension ranges from 25-75% [26]. It is well known that preloading colloids before spinal anesthesia significantly reduces the incidence of hypotension and related side effects compared to crystalloids. Thus, colloids are a better fluid for pre-hydration than crystalloids [27]. Although we did not analyze patient hemodynamics such as spinal anesthesia-induced hypotension or hypotension during the surgery as a secondary outcome, we believe that the result of this study in which preloading of 6% HES-130/0.4 decreased the amount of intraoperative fluid is worth referring to in the future.
This study had several limitations. First, we included only patients with well-controlled diabetes (HbA1c < 7.0%) who took oral medications. Postoperative hyperglycemia in patients with diabetes is significantly associated with preoperative fasting glucose levels and HbA1c [28]. Therefore, diabetic patients with poor glycemic control are vulnerable to surgical stress and may be at a risk of increased postoperative morbidity and mortality [6]. Therefore, the results may differ if the experiment were to be conducted in patients with poor glycemic control. Furthermore, additional research that includes patients with glycemic control using insulin is required. Second, although it is known that the use of lactate Ringer’s solution leads to transient hyperglycemia via the conversion of lactate to glucose in diabetic patients [29], Ringer’s lactate solution was used as the control fluid in this study. However, according to a theoretical analysis, one liter of lactated Ringer’s solution contains 29 mmol of lactate, which can create a maximum increase in glucose concentration by about 18.02 mg/dl in a patient weighing 70 kg and that has 100% gluconeogenesis efficiency [30]. Moreover, lactate alone cannot induce significant hyperglycemia in actual clinical practice because the efficiency of gluconeogenic pathways is not 100% and the loss of lactate is due to oxidative metabolism [30]. Therefore, the use of lactated Ringer’s solution as a control fluid seems to have a limited effect on glycemic control in fasting diabetic patients under spinal anesthesia. Finally, the sample size of the current study was relatively small, despite it being deemed as appropriate based on the calculations for the pilot study. In addition, we only observed glucose levels 4 h after the administration of fluids. As approximately half of the administered HES remains after 24 h [7,9], a long-term follow-up study with a larger sample size is needed.
In conclusion, the preloading of 6% HES-130/0.4 in patients with diabetes under spinal anesthesia did not increase blood glucose levels compared to lactate Ringer’s solution. There were also no significant differences in the serial changes in mean blood glucose levels after administration of fluids between 6% HES-130/0.4 and lactate Ringer’s solution. Therefore, evidence that 6% HES-130/0.4 produces hyperglycemia in diabetic patients could not be found; however, further evaluation with long-term follow-up is required.
Notes
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Tae Hun An. Data curation: Soo Yeon Cho, Soo Bin Shim, Myungjin Lee, Ki Tae Jung. Formal analysis: Soo Yeon Cho, Ki Tae Jung. Funding acquisition: Ki Tae Jung. Methodology: Tae Hun An. Writing - original draft: Soo Yeon Cho, Soo Bin Shim, Ki Tae Jung. Writing - review & editing: Tae Hun An, Myungjin Lee, Ki Tae Jung. Investigation: Soo Bin Shim, Ki Tae Jung. Resources: Tae Hun An. Supervision: Tae Hun An, Ki Tae Jung. Validation: Tae Hun An.
Fig. 1.
Consort flow diagram for the study. Group L was preloaded with lactated Ringer’s solution (15 ml/kg) as the control group. Group H was preloaded with 6% hydroxyethyl starch 130/0.4 (7.5 ml/kg).
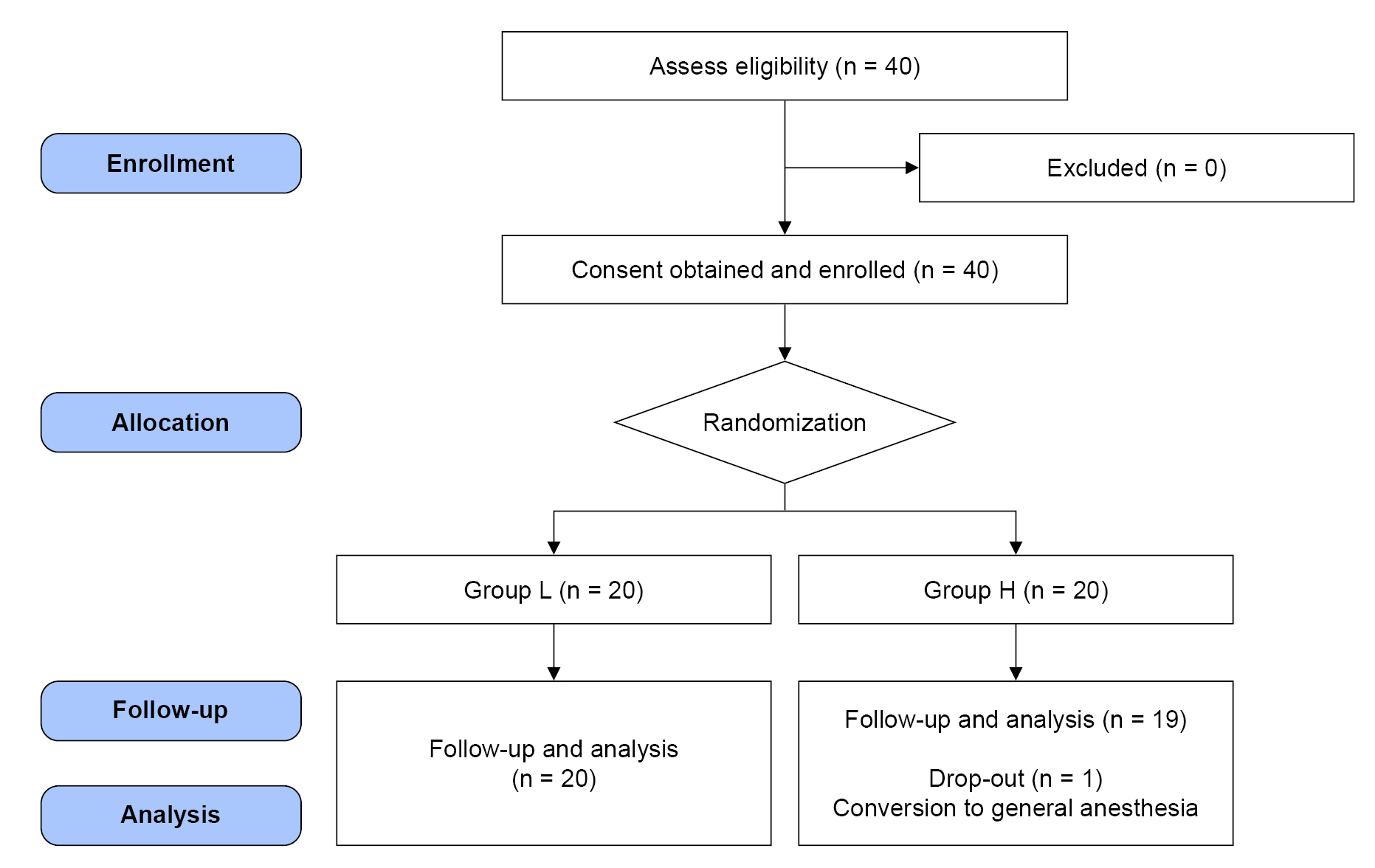
Fig. 2.
Changes in the mean blood glucose levels (mean ± SD). The numbers represent the changes in mean blood glucose levels from the baseline values (T0) at certain time points (mean ± SD). Group L was preloaded with lactated Ringer’s solution (15 ml/kg) as the control group, whereas Group H was preloaded with 6% hydroxyethyl starch 130/0.4 (7.5 ml/kg). T0: baseline before administration of the designated fluid, T1: 30 minutes after administration of the designated fluid, T2: 1 hour after administration of the designated fluid, T3: 2 hours after administration of the designated fluid, T4: 3 hours after administration of the designated fluid.
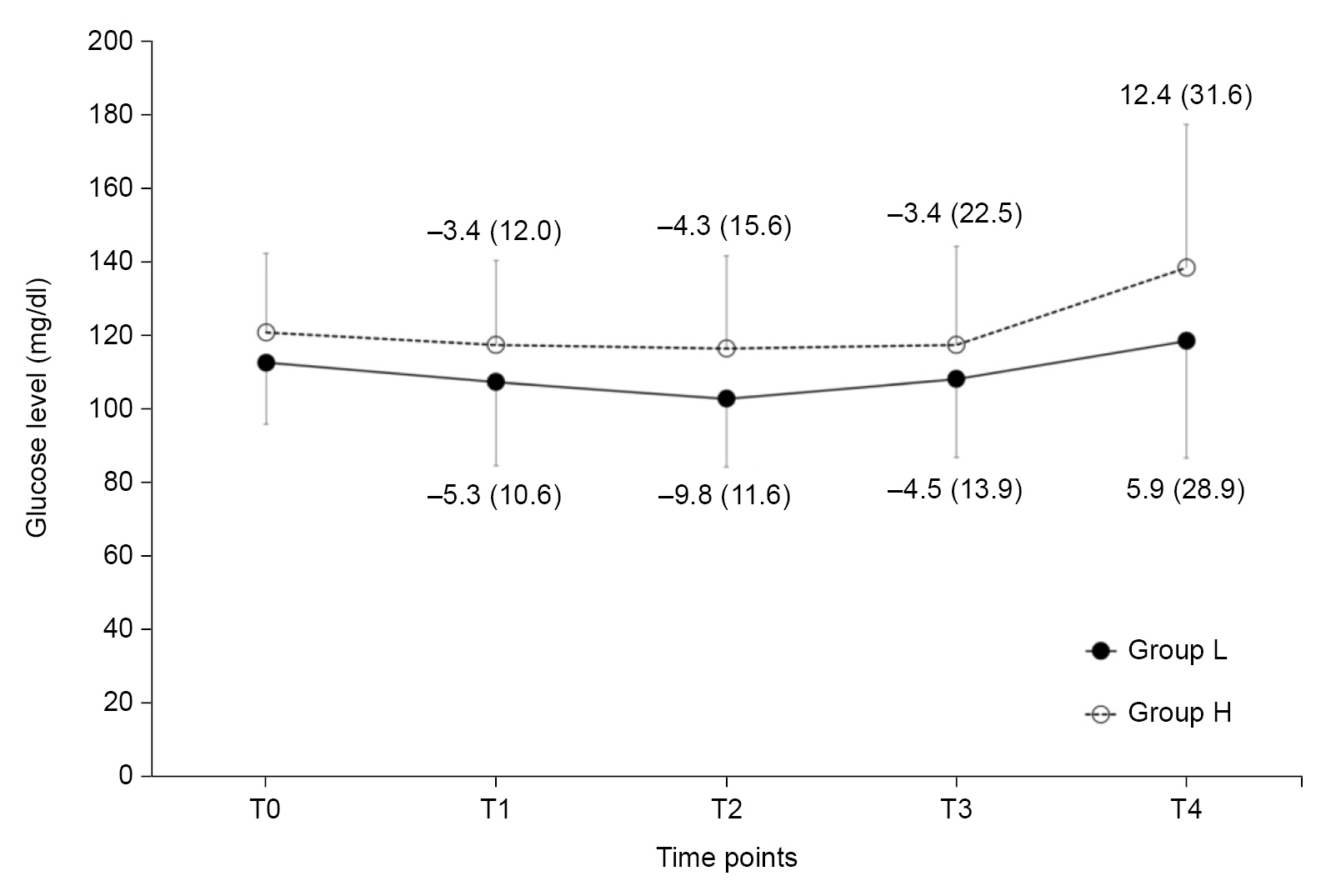
Fig. 3.
Changes in mean blood pressure and heart rate (mean ± SD). (A) Mean blood pressure and (B) heart rate. Group L was preloaded with lactated Ringer’s solution (15 ml/kg) as the control group, whereas Group H was preloaded with 6% hydroxyethyl starch 130/0.4 (7.5 ml/kg). T0: baseline before administration of the designated fluid, T1: 30 minutes after administration of the designated fluid, T2: 1 hour after administration of the designated fluid, T3: 2 hours after administration of the designated fluid, T4: 3 hours after administration of the designated fluid.

Table 1.
Patient Characteristics and Preoperative Variables
Table 2.
Perioperative Outcomes
REFERENCES
1. Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: an update. Anesthesiology 2017; 126: 547-60.

2. Seo KH. Perioperative glucocorticoid management based on current evidence. Anesth Pain Med (Seoul) 2021; 16: 8-15.



3. Vriesendorp TM, Morélis QJ, Devries JH, Legemate DA, Hoekstra JB. Early post-operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study. Eur J Vasc Endovasc Surg 2004; 28: 520-5.


4. Cheisson G, Jacqueminet S, Cosson E, Ichai C, Leguerrier AM, Nicolescu-Catargi B, et al. Perioperative management of adult diabetic patients. Intraoperative period. Anaesth Crit Care Pain Med 2018; 37 Suppl 1: S21-5.
5. Lee SY, Lee KM, Lee JG, Lim JA, Woo NS, Lee YC. Effect of hyperglycemia on the length of postoperative hospital stay. Korean J Anesthesiol 2005; 48: 565-9.

6. Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010; 33: 1783-8.



7. Jeon YS. Recent findings on the use of hydroxyethyl starch. Anesth Pain Med 2014; 9: 159-64.
8. Myburgh JA. Fluid resuscitation in acute medicine: what is the current situation? J Intern Med 2015; 277: 58-68.


9. Jungheinrich C, Neff TA. Pharmacokinetics of hydroxyethyl starch. Clin Pharmacokinet 2005; 44: 681-99.


10. Buggy D, Higgins P, Moran C, O'Brien D, O'Donovan F, McCarroll M. Prevention of spinal anesthesia-induced hypotension in the elderly: comparison between preanesthetic administration of crystalloids, colloids, and no prehydration. Anesth Analg 1997; 84: 106-10.

11. Chawnchhim AL, Jacob M, Jaiswal A, Paul D, Kaur KB, Ray A, et al. Effect of hydroxyethyl starch on blood sugar levels in surgeries under subarachnoid block: a cross-sectional study. J Clin Diagnostic Res 2020; 14: UC11-4.

12. Jung KT, Shim SB, Choi WY, An TH. Effect of hydroxyethyl starch on blood glucose levels. Korean J Anesthesiol 2016; 69: 350-6.



13. Lou S, Bian L, Long C, Wang Z, Ma J, Zhou B. Does 6% hydroxyethyl starch 130/0.4 impact differently on blood glucose than 4% gelatin in patients receiving open heart surgery? Perfusion 2012; 27: 113-8.


14. Murty SS, Kamath SK, Chaudhari LS. Effects of hydroxyethyl starches on blood sugar levels: a randomized double blind study. Indian J Anaesth 2004; 48: 196-200.
15. Patki A, Shelgaonkar V. Effect of 6% hydroxyethyl starch-450 and low molecular weight dextran on blood sugar levels during surgery under subarachnoid block: a prospective randomised study. Indian J Anaesth 2010; 54: 448-52.



16. Chandra AP. Effects of preloading with isotonically balanced tetra hydroxyethyl starch versus preloading with gelufusine on blood glucose level in diabetic patients. Acad Anesthesiol Int 2020; 5: 162-7.
18. Sudhakaran S, Surani SR. Guidelines for perioperative management of the diabetic patient. Surg Res Pract 2015; 2015: 284063.



19. American Diabetes Association. 14. Diabetes care in the hospital: standards of medical care in diabetes-2018. Diabetes Care 2018; 41(Suppl 1): S144-51.


20. Kang H. Sample size determination for repeated measures design using G Power software. Anesth Pain Med 2015; 10: 6-15.

22. Gottschalk A, Rink B, Smektala R, Piontek A, Ellger B, Gottschalk A. Spinal anesthesia protects against perioperative hyperglycemia in patients undergoing hip arthroplasty. J Clin Anesth 2014; 26: 455-60.


23. Baron JF. A new hydroxyethyl starch: HES 130/0.4 Voluven®. Transfus Altern Transfus Med 2000; 2: 13-21.

24. Kirch W, Köhler H, Horstmann HJ. Retarded elimination of a high-molecular enzyme-substrate-complex after hydroxyethyl-starch-infusion. editors. Toxicological aspects of food safety. In: Leonard BJ, Berlin, Springer. 1978. p. 335-8.
25. Yadav R, Bhartiya JP, Verma SK, Nandkeoliar MK. The evaluation of serum amylase in the patients of type 2 diabetes mellitus, with a possible correlation with the pancreatic functions. J Clin Diagn Res 2013; 7: 1291-4.



26. Bajwa SJ, Kulshrestha A, Jindal R. Co-loading or pre-loading for prevention of hypotension after spinal anaesthesia! A therapeutic dilemma. Anesth Essays Res 2013; 7: 155-9.



27. Kim CS, Ahn HJ, Shin SH, Choi DH. Prevention of hypotension with crystalloid versus colloid during spinal or combined spinal-epidural anesthesia for cesarean delivery. Korean J Anesthesiol 2004; 46: 408-13.

28. Mannion JD, Rather A, Manifold S, Gardner K, McEvilly M, Yaeger J, et al. Postoperative hyperglycemia in patients with and without diabetes after major joint replacement: the impact of an enhanced glucose management program. JB JS Open Access 2021; 6: e20.00172.


-
METRICS

-
- 1 Crossref
- 1,849 View
- 58 Download
- Related articles in Anesth Pain Med
- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others







