Post-dural puncture headache prevention and treatment with aminophylline or theophylline: a systematic review and meta-analysis
Article information
Abstract
Background
Post-dural puncture headache (PDPH) is one of the most common complications in patients undergoing spinal anesthesia. The present systematic review and meta-analysis aimed to assess the therapeutic and prophylactic effects of aminophylline and theophylline on PDPH.
Methods
Relevant studies were identified by searching the following electronic databases, without language restriction, until June 2020: Scopus, EMBASE, MEDLINE, Google Scholar, Web of Science, Cochrane Library-CENTRAL, and CINAHL Complete. Random effects models were used to calculate the standardized mean difference (SMD) and risk ratios (RRs) with 95% confidence intervals (95% CI) to assess the therapeutic and prophylactic effects of aminophylline and theophylline on PDPH, respectively. The Cochrane tool was used for the quality assessment of the included studies. The certainty of the evidence was rated using the Grading of Recommendations Assessment, Development, and Evaluation method.
Results
Of the 1,349 initial records, 15 met our eligibility criteria (6 studies on therapeutic and 9 on prophylactic effects). A significant reduction in the pain score was observed following aminophylline/theophylline treatment (SMD = –1.67; 95% CI, –2.28 to –1.05; P < 0.001, I2 = 84.7%; P < 0.001). Subgroup analysis revealed that the therapeutic effect was significantly higher when these agents were compared to placebo than when conventional therapies were used. The risk of PDPH after aminophylline administration was not significantly reduced (RR = 0.74; 95% CI, 0.42 to 1.31; P = 0.290).
Conclusions
Theophylline and aminophylline have therapeutic, but not prophylactic, effects on PDPH.
INTRODUCTION
Lumbar puncture is a surgical procedure used primarily to sample cerebrospinal fluid (CSF) or to inject medications, including anesthetics [1-3]. Post-dural puncture headache (PDPH) is one of the most common complications observed in patients undergoing lumbar puncture and is usually accompanied by photophobia, nausea, neck stiffness, and subjective hearing symptoms [4]. During lumbar puncture, the size, shape, and orientation of the spinal needles and the patient’s position can affect the likelihood of developing PDPH. There are also factors associated with PDPH risk, including age (higher risk for patients between 18 and 40 years), female sex, low body mass index, chronic headaches, and previous PDPH history [5]. The incidence of PDPH after spinal anesthesia and lumbar puncture (with a standard traumatic needle) is < 3% and 11%, respectively [4].
The epidural blood patch is considered an effective strategy for PDPH prevention in high-risk patients and for PDPH treatment in severe or debilitating forms. Nonetheless, there are several concerns regarding its application owing to its invasiveness, need for anesthesia practitioners, and questionable efficacy [5]. Furthermore, lying down, drinking plenty of fluids, and non-steroidal anti-inflammatory drugs are routinely recommended for PDPH prevention after dural puncture [6]. However, there is poor evidence regarding the efficacy of these recommendations compared with immediate mobilization [7]. Therefore, additional clinical studies are needed to identify effective pharmacological options for PDPH prevention or treatment [8].
Methylxanthines are purine alkaloids mainly known for their bronchodilator and stimulatory effects. Some previous studies have reported conflicting evidence on the effects of methylxanthine derivatives, including aminophylline and theophylline, on PDPH [9-11]. A recent meta-analysis investigated the impact of methylxanthines on the incidence and severity of PDPH. In this study, 10 randomized controlled trials (RCTs) were analyzed, and a significant therapeutic effect of aminophylline against PDPH was reported [12]. Therefore, our study sought to better understand the effect of aminophylline and theophylline on PDPH prevention and treatment through a more comprehensive search of multiple electronic databases, without language restriction, and to evaluate the certainty of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).
MATERIALS AND METHODS
Search strategy
The protocol for this systematic review and meta-analysis was written using the guidelines of the Cochrane Handbook, registered in the International Prospective Register of Systematic Reviews (registration number: CRD42020211990), and published in a peer-reviewed journal [13]. Databases of EMBASE, Scopus, Google Scholar, PubMed/MEDLINE, Web of Science, the CINAHL Complete, and Cochrane Library-CENTRAL were searched between January 1, 1980, and June 30, 2020, using the following key search terms: “post-dural puncture headache,” “headache,” “post lumbar puncture headache,” “aminophylline,” and “theophylline.” No restrictions on publication language or study type were applied. A complete search strategy is available in a previously published protocol [13]. Moreover, the reference lists of all relevant studies, theses, proceedings, and conference papers were sought to ensure that all eligible studies were included.
Study selection
In the first screening stage, two authors (RBB and SSZ) reviewed the titles and abstracts of the papers using a checklist. The final selection of studies was carried out independently by two contributors (RBB and MM), following a review of the full texts of the studies obtained during the screening phase. Disputes between these reviewers were resolved by consensus or by a third expert’s opinion (ARS). The following studies were included in the current research: 1) trials on the preventive or therapeutic effects of intravenous or oral administration of aminophylline/theophylline on PDPH compared to placebo or conventional therapy (complete bed rest, hydration, acetaminophen codeine, and pethidine), 2) studies on participants (male, female, or both sexes) who underwent lumbar puncture before surgery (all surgeries); and 3) interventions evaluating the incidence or severity of PDPH pre- and post-intervention, as our primary outcome. Furthermore, our secondary outcomes were the assessment of aminophylline/theophylline effects based on the participants’ characteristics, type of control and intervention, route of intervention administration, and methodological quality of the studies and finding the source of heterogeneity accordingly.
Studies were excluded if they were in the pediatric population or did not provide information regarding outcomes.
Quality assessment
Cochrane’s tool was used to evaluate the methodological quality of the studies [14]. Accordingly, we considered random sequence generation, allocation concealment, blinding of personnel, participants, outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias in each study. The overall risk of bias was rated as “high,” “low,” or “moderate.”
Data extraction
Three authors (RBB, SSZ, and MM) assessed eligible studies. In cases of disagreement, discussion between reviewers or the fourth expert’s opinion (ARS) was used to reach a consensus. We extracted the following information from each study: name of the first author, publication year, country, study design and location, participants’ characteristics, sample size, quality of paper, type of operation, headache scale, dosage of intervention, type of comparison arm, mean and standard deviation (SD) of pain scores in both groups, and the number of patients with and without headache in both intervention and control groups. The authors independently calculated the required data for the included studies or contacted the study authors to collect data if the included studies had incomplete data. Papers were excluded if the authors did not respond to the queries three times. Data extraction was performed using Web Plot Digitizer software when the outcome variables were reported only by graphs.
Quantitative data synthesis and statistical analysis
Statistical analyses were performed using STATA version 13.1 (StataCorp, USA). Cohen’s Kappa statistics were used to assess inter-agreement scores between reviewers during the selection process (0.895). We calculated the risk ratios (RRs) for dichotomous data using the random effects model and Mantel-Haenszel method with 95% confidence intervals (CIs). The standardized mean difference (SMD) and 95% CI were also applied to evaluate the effect of aminophylline/theophylline on PDPH severity. We extracted the mean and SD of the visual analog scale (VAS) or numerical rating scale (NRS) before and after the trial. To compute the mean change, we used the following formula: the amount at the end of the study minus the baseline amount in the treatment and control groups. The SD of the mean difference was computed as follows (if not reported): SD = square root [(SD pre-treatment)2 + (SD post-treatment)2 – (2 R × SD pre-treatment × SD post-treatment)], assuming 0.5 as a conservative estimate for R [14]. The procedure of Hozo et al. [15] was also used to estimate the mean and SD values when the median and range or 95% CIs were reported.
We used the Q-statistic and I2 statistic tests to investigate the statistical heterogeneity. Heterogeneity levels of 0–40%, 30–60%, 50–90%, and 75–100% were categorized as “probably not significant,” “moderate heterogeneity,” “substantial heterogeneity,” and “considerable heterogeneity,” respectively [16].
For the therapeutic effects of aminophylline/theophylline on PDPH, subgroup analysis was performed based on age (< 32 and ≥ 32 years), type of control group (placebo and conventional therapy), time of pain assessment after aminophylline/theophylline consumption (≤ 12 and > 12 h), route of treatment administration (intravenous and oral), type of intervention (theophylline and aminophylline), and quality of studies (low and moderate vs. high). In terms of prophylactic effect, subgroup analysis was considered based on age (< 32 and ≥ 32 years), study population (patients undergoing cesarean section and lower extremity surgery), and quality of studies (high and moderate).
A sensitivity analysis was conducted using the leave-one-out method to evaluate the effect of each study on the overall effect size. Meta-regression was performed to assess the association between effect size, age, and time of pain assessment following aminophylline treatment. Funnel plot, Begg’s test, and Egger’s test were used to identify potential publication bias when an outcome was assessed in 10 or more studies. Statistical significance was set at P < 0.05.
Certainty of the evidence
The certainty of the evidence was assessed based on the GRADE approach. Each outcome was scored as high, moderate, low, or very low. There were five domains for downgrading (risk of bias, inconsistency, indirectness, imprecision, and publication bias) and three criteria for upgrading outcomes (large magnitude of association, dose–response gradient, and residual plausible bias and confounding) [17].
RESULTS
The selection process for the meta-analysis is illustrated in Fig. 1. First, 2019 reports were identified. After eliminating duplicates, 1,049 articles remained. Based on titles and abstracts, 1,028 articles were excluded. Thus, 21 potentially relevant articles were selected and examined in detail. Finally, six studies were excluded for one or more of the following reasons: non-RCT trial (n = 2) [18,19]; conference abstract, with no available data (n = 1) [20]; inappropriate data (n = 2) [21]; and use of aminophylline in combination with other components without a suitable control group (n = 1) [22]. After ultimate evaluation, 15 eligible studies (6 on therapeutic and 9 on prophylactic effects) met the inclusion criteria and were appropriate for inclusion in the final meta-analysis.
Risk of bias assessment
Supplementary Figs. 1 and 2 show the risk of bias in individual trials and the risk of each bias among all trials, respectively. Eight studies described the method of assigning participants to the intervention and control groups by random sequence generation [10,11,19,23-27], whereas the others did not provide a complete explanation. Allocation concealment was reported in only three studies [1,28,29] and four trials were not blinded [26,27,29-31]. No high risk of attrition bias was detected in the included studies. Except for one study [32], the other studies were judged to be at low risk for selective reporting. Ultimately, eight studies were deemed to be at low risk [1,10,11,23-25,28,33], three at high risk [30-32], and four at unclear risk of bias [26,27,29,34].
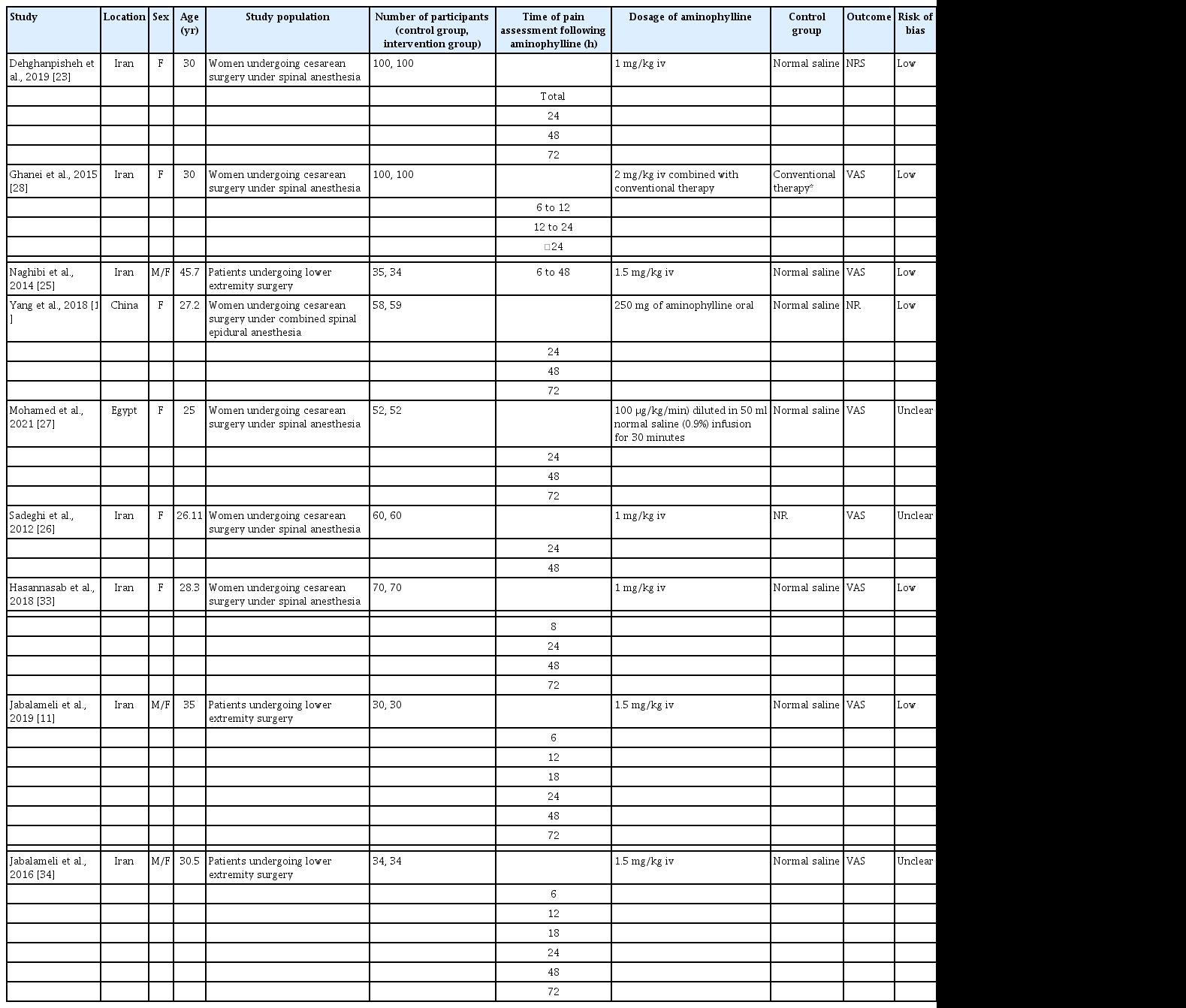
Characteristics of the included studies
Table 1 presents the characteristics of the included studies. Data on the therapeutic effects of aminophylline or theophylline on PDPH were obtained from six eligible studies, including 195 and 194 participants in the control and intervention groups, respectively (four studies on theophylline and two on aminophylline effects). These trials included 17 [31] to 62 [1] participants. These studies were published between 2007 and 2021 and were conducted in Iran (one study) [32], Egypt (two studies) [24,29], China (one study) [1], Turkey (one study) [31], and India (one study) [30]. The participants’ mean age ranged from 26.23 [29] to 40.06 [32] years. All trials were conducted for both sexes. The duration of the intervention ranged from 4 [31] to 24 h [30].

Characteristic of Studies That Evaluated the Therapeutic Effect of Aminophylline/theophylline on PDPH
Regarding the prophylactic impact of aminophylline on PDPH (Table 2), nine studies with sample sizes varying from 60 to 200 were evaluated. Six of these studies focused on participants who underwent cesarean sections [10,23,26-28,33], and three assessed subjects undergoing extremity surgeries [11,25,34]. The included studies were published between 2014 and 2021 and conducted in Iran (seven studies) [11,23,25,26,28,33,34], Egypt (one study) [27], and China (one study) [10]. The mean age of the participants ranged from 25 [27] to 45.7 [25] years.
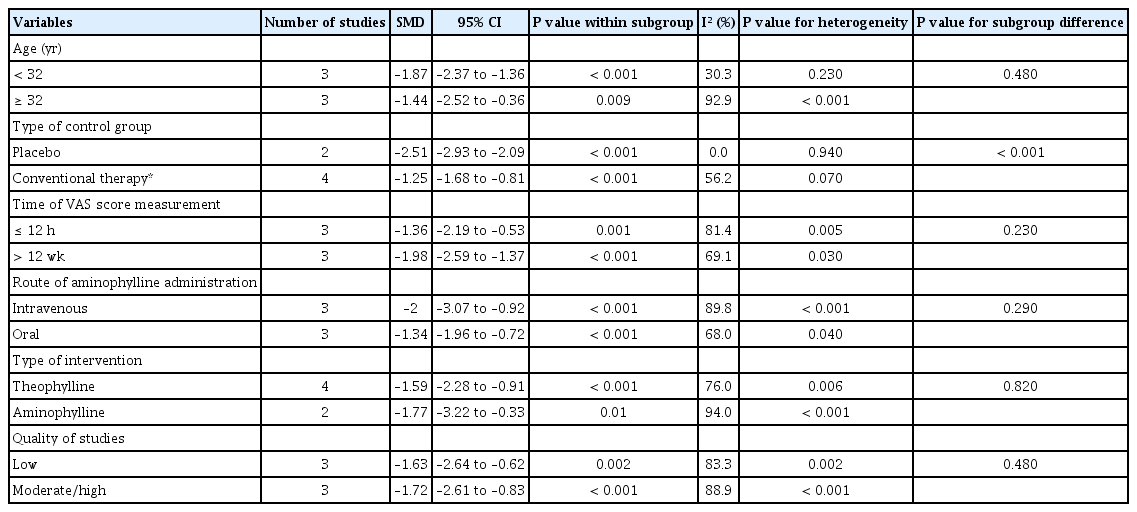
Meta-analysis and subgroup results of the therapeutic effect of aminophylline/theophylline on PDPH
Fig. 2 shows a significant reduction in VAS or NRS pain scores in the aminophylline/theophylline group compared to the placebo or conventional therapy control group (SMD = –1.67; 95% CI, –2.28 to –1.05; P < 0.001), with significant heterogeneity (I2 = 84.7%; P < 0.001).

Forest plot displaying standard mean difference (SMD) and 95% confidence intervals (CIs) for the therapeutic impact of aminophylline on severity of post-dural puncture headache compared with placebo or conventional therapy as a control group.
Table 3 shows the results of the subgroup analysis. When the data were sub-grouped by the type of control group, the pooled effect size was significantly different between studies using placebo (SMD = –2.51; 95% CI, –2.93 to –2.09; P < 0.001) and conventional therapy (SMD = –1.25; 95% CI, –1.68 to –0.81; P < 0.001). There were no significant differences between the subgroups in age (P = 0.48), time of VAS score assessment (P = 0.230), type of intervention (P = 0.820), route of drug administration (P = 0.290), and quality of studies (P = 0.480). Moreover, the heterogeneity was significantly reduced following subgroup analyses by age (I2 = 30.3%; P = 0.230) and type of control group (I2 = 0.0%; P = 0.940).
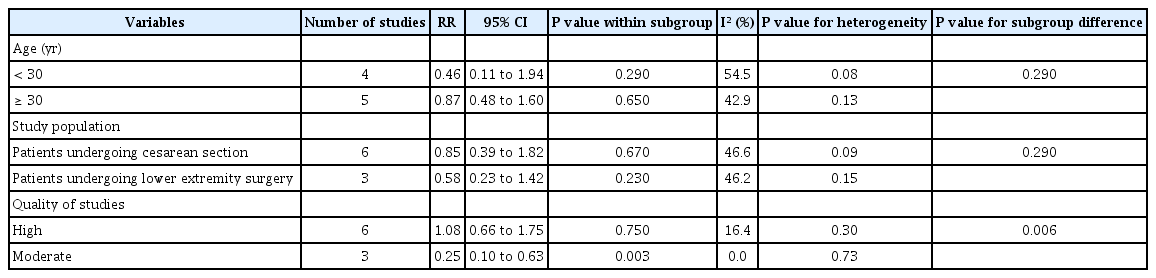
Meta-analysis and subgroup results of the prophylactic effect of aminophylline on PDPH
Fig. 3A shows the prophylactic effect of aminophylline on PDPH at all time points after its administration (nine studies, 1,078 participants) [10,11,23,25-28,33,34]. Moreover, Fig. 3 panels B, C, and D demonstrate the prophylactic effect of aminophylline against PDPH at 24 h (eight studies, 1,009 participants) [10,11,23,26-28,33,34], 48 h (eight studies, 787 participants) [10,11,23,25-27,33,34], and 72 h after aminophylline administration (six studies, 689 participants) [10,11,23,27,33,34], respectively.
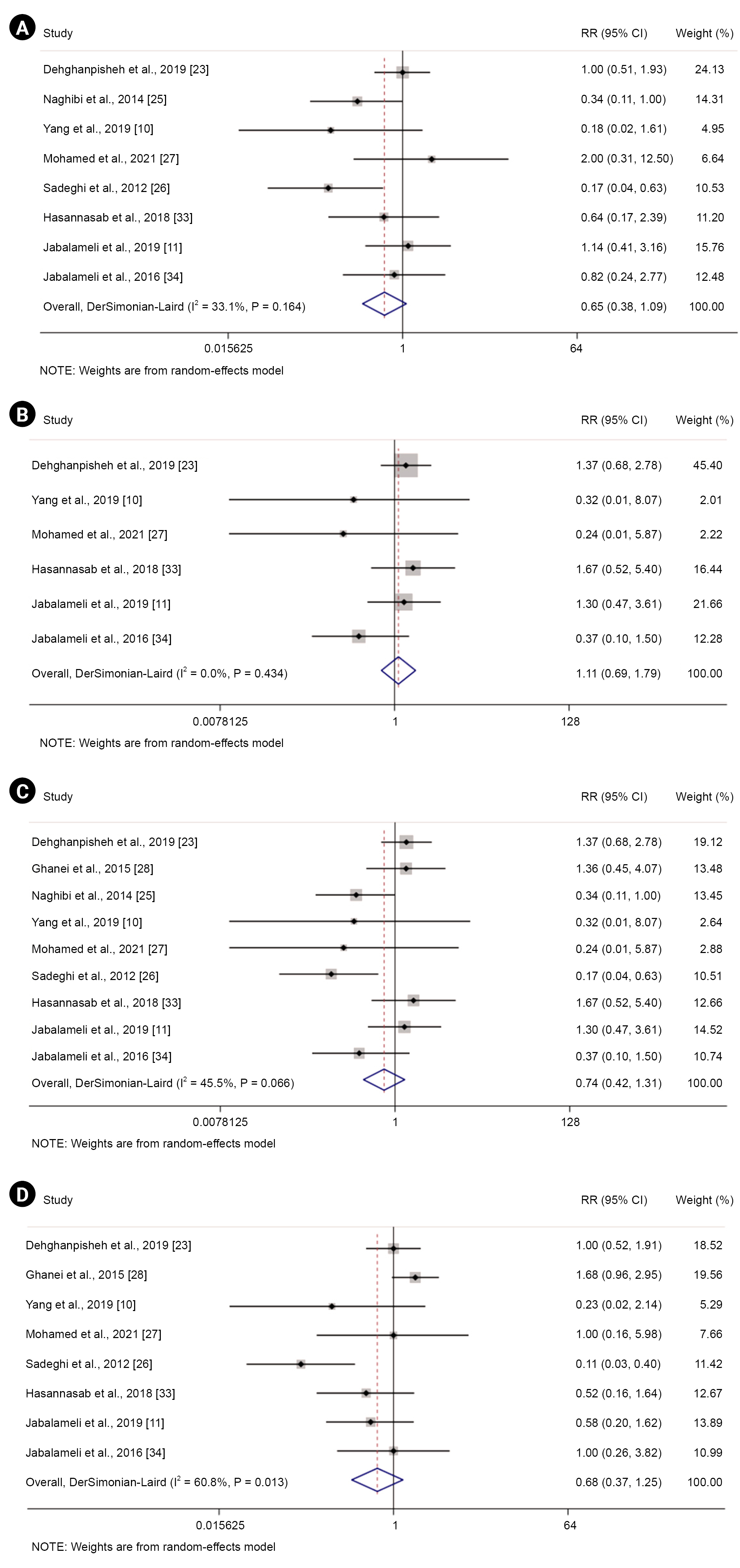
Forest plot displaying risk ratio (RR) and 95% confidence intervals (CIs) for the prophylactic impact of aminophylline on post-dural puncture headache compared with a control group (A) and after 24 (B), 48 (C), and 72 h (D).
Our results revealed a non-significant reduction in the risk of PDPH at all time points (RR = 0.74; 95% CI, 0.42 to 1.31; P = 0.290, I2 = 45.5%; P = 0.07), 24 h (RR = 0.68, 95% CI, 0.37 to 1.25; P = 0.210, I2 = 60.8%), and 48 h after aminophylline administration (RR = 0.65, 95% CI, 0.38 to 1.09; P = 0.100, I2 = 33.1%). Nonetheless, a non-significant increase was observed in the risk of PDPH at 72 h following aminophylline administration (RR = 1.11, 95% CI, 0.69 to 1.79; P = 0.650, I2 = 0%).
As shown in Table 4, there were no significant differences in the subgroup analyses stratified by age (P = 0.290) and study population (P = 0.290). Nonetheless, a significant difference was observed in the risk of PDPH between moderate-quality (RR = 0.25; 95% CI, 0.10 to 0.63; P = 0.003) and high-quality studies (RR = 1.08; 95% CI, 0.66 to 1.75; P = 0.750). Furthermore, heterogeneity also decreased in this subgroup (I2 = 0%; P = 0.730).
Sensitivity analysis and publication bias
Neither trial had a significant influence on the summary effect in either of the analyses. As our outcomes were reported in less than ten studies, publication bias tests were not performed.
Meta-regression
Meta-regression was performed for the participants’ age and time of pain assessment following aminophylline administration. None of the two factors had a significant effect on pain score (t = 0.44; 95% CI, –0.17 to 0.23 and t = –1.11; 95% CI, –0.07 to 0.02; respectively) (Supplementary Fig. 3).
Certainty of the evidence
The overall certainty of the evidence was moderate and very low for the therapeutic and prophylactic effects of theophylline/aminophylline on PDPH, respectively (Supplementary Table 1).
DISCUSSION
In the present study, we investigated the therapeutic effects of theophylline and aminophylline on PDPH. This impact was significantly higher when these methylxanthines were compared with placebo than with conventional therapies. Furthermore, we observed no prophylactic effects of aminophylline on PDPH. The quality of the studies, type of control group, and age were detected as sources of heterogeneity in the present study.
PDPH is one of the most common complications of lumbar puncture, which is usually accompanied by nausea, vomiting, stiff neck, hearing loss, tinnitus, and photophobia and may influence the quality of life and hospital discharge of patients [19]. There remains a great deal to learn regarding the pathophysiology of PDPH [35]. This headache is primarily due to a CSF leak following a dural puncture with resultant intracranial hypotension and, subsequently, downward traction on pain-sensitive intracranial structures caused by gravity in the standing position. In contrast, intracranial volume restoration by dilating cerebral blood vessels as a compensatory adaptation may further exacerbate PDPH symptoms [5].
Different interventions are available to reduce numerous types of pain, such as PDPH, with varying degrees of success and risks [36-38]. Bed rest, intravenous hydration, and analgesic medications are conservative therapies for PDPH. Theophylline and its salt formulation (aminophylline) are methylxanthines with proposed therapeutic effects in PDPH. According to the present systematic review and meta-analysis, theophylline/aminophylline was effective in reducing pain severity after PDPH onset. These methylxanthines are superior to acetaminophen [24], conservative treatment (comprising caffeine) [30], and placebo for pain relief in PDPH. Furthermore, no adverse effects were reported following aminophylline/theophylline administration in these patients [10,29,30]. These results are in line with those of a previous meta-analysis of five studies reporting a lower pain score in patients receiving aminophylline (MD = –1.34; 95% CI, –1.76 to –0.91). Nonetheless, a comparison between aminophylline and conservative treatments was not conducted in this study [12].
Multiple prophylactic strategies for PDPH have been studied, but their clinical effectiveness has not yet been established. Bed rest and hydration are routinely recommended by clinicians for the prevention of PDPH following dural puncture. Nonetheless, according to a Cochrane review, there is no evidence to support the benefits of routine bed rest and fluid supplementation in preventing PDPH onset [7]. In the present meta-analysis, we observed no beneficial effect of theophylline/aminophylline on the risk of PDPH. However, subgroup analysis revealed prophylactic effects in moderate-quality studies. In another meta-analysis, Hung et al. [12] demonstrated no significant preventive effect of aminophylline against PDPH at 24, 48, or 72 h. However, subgroup analysis was not performed to assess the impact of several factors, including study quality, on the findings.
Aminophylline is suggested to have pain-relief effects in PDPH through cerebral vasoconstriction by interfering with calcium uptake by the sarcoplasmic reticulum of endothelial cells, blocking phosphodiesterase, increasing the intracellular cyclic adenosine monophosphate concentration, contraction of intracranial blood vessels by antagonizing adenosine function, increasing CSF secretion by stimulating sodium and potassium pumps, and blocking the transmission of pain perception [19].
The strengths of the present systematic review and meta-analysis are the search for several electronic databases without language restriction, performing meta-regression, and conducting subgroup analysis to detect sources of heterogeneity and the effects of subgroups on the pooled estimates. However, considerable between-study statistical and methodological heterogeneity and the low number of included studies, most of which were performed in Asia or were of low quality, are the major limitations of the present study. Therefore, the findings should be interpreted with caution. Further studies are needed to confirm these results because of the moderate and very low certainty of the present evidence.
According to the present systematic review and meta-analysis, theophylline and aminophylline had analgesic effects on PDPH. Nonetheless, we observed no prophylactic effect of aminophylline on PDPH.
SUPPLEMENTARY MATERIALS
Supplementary data including a questionnaire results of risk of bias assessment, meta-regression, and certainty of evidence can be found online at https://doi.org/10.17085/apm.22247.
Certainty of Evidence according to GRADE Approach
An overview of the authors’ judgments about the risk of bias in each study.
The overall risk of bias of the included studies.
Meta-regression plots of the association between mean changes in severity of post-dural puncture headache with mean age of participant (A) and time of VAS score measurement (hours) (B). VAS: visual analog scale.
Notes
FUNDING
This study was supported by the Vice Chancellor for Research and Technology of Shiraz University of Medical Sciences (code: 21331). The funder had no role in the study design, analysis, decision to publish, or manuscript preparation.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Reza Barati-Boldaji, Sara Shojaei-Zarghani, Manoosh Mehrabi, Afshin Amini, Ali Reza Safarpour. Data curation: Reza Barati-Boldaji, Ali Reza Safarpour. Formal analysis: Reza Barati-Boldaji, Ali Reza Safarpour. Funding acquisition: Ali Reza Safarpour. Methodology: Reza Barati-Boldaji, Sara Shojaei-Zarghani, Manoosh Mehrabi, Afshin Amini, Ali Reza Safarpour. Project administration: Ali Reza Safarpour. Visualization: Ali Reza Safarpour. Writing - original draft: Reza Barati-Boldaji, Sara Shojaei-Zarghani. Writing - review & editing: Manoosh Mehrabi, Afshin Amini, Ali Reza Safarpour. Investigation: Ali Reza Safarpour. Resources: Ali Reza Safarpour. Software: Ali Reza Safarpour. Supervision: Ali Reza Safarpour. Validation: Ali Reza Safarpour.
Acknowledgements
Our sincere gratitude goes to the Vice-Chancellor for Research at Shiraz University of Medical Sciences for approving this systematic review and meta-analysis (ID: 21331).