INTRODUCTION
Postpartum hemorrhage (PPH) is a leading cause of maternal morbidity and mortality [
1]. Therefore, it is important to prevent PPH. The identification of risk factors associated with PPH is essential to prevent the development of minor hemorrhages into severe bleeding. The American College of Obstetricians and Gynecologists defines PPH as cumulative blood loss Ōēź 1,000 ml or blood loss accompanied by signs or symptoms of hypovolemia that develop within 24 h after the birth process (including intrapartum blood loss), regardless of the delivery method [
2].
Placental disorders, general anesthesia, previous history of curettage or cesarean delivery, twin pregnancy, and gestational diabetes or hypertension are known risk factors associated with PPH after cesarean delivery [
3-
5]. A recent retrospective study reported that chronic and gestational diabetes, magnesium administration, anemia or thrombocytopenia on hospitalization, and general anesthesia are risk factors for the development of PPH during twin delivery. This risk was regardless of the delivery method; however, PPH was more commonly observed after cesarean delivery compared to that after vaginal delivery [
6].
The number of parturients with twin fetuses has been increasing due to the development and growth of assisted reproductive therapy technology techniques [
7]. Therefore, it is important to understand the PPH risk factors associated with cesarean delivery for twin pregnancy. To date, very few studies have assessed the risk of PPH in twin fetal cesarean deliveries worldwide [
6,
8]. Moreover, no such studies have been conducted in Korea. Therefore, this nationwide Korean study evaluated the risk factors for severe PPH during the peripartum period in women who underwent cesarean delivery for twin fetal pregnancy. In this study, severe PPH was defined as excessive bleeding requiring red blood cell (RBC) transfusion after cesarean delivery for twin pregnancy [
9]. Korean Health Insurance Review and Assessment Service (HIRA) data, which could be considered as national data, was used to evaluate the risk factors.
MATERIALS AND METHODS
The study protocols were approved by the Institutional Review Board of CHA Bundang Medical Center, CHA University (approval no. CHAMC 2021-09-018; approval date: Oct 01, 2021). The study was registered with the Clinical Research Information Service (
https://cris.nih.go.kr, Registration No. KCT0006675; registration date: Oct 19, 2021). The claims data of parturients were obtained from HIRA. HIRA of Korea contains records of cesarean deliveries performed by the Korean National Health Insurance Service for Korean citizens and some foreigners who have resided in Korea for a long duration. Clinical features and comorbidities were confirmed using the Korean Classification of Diseases, which was based on the 10th edition of the International Classification of Diseases.
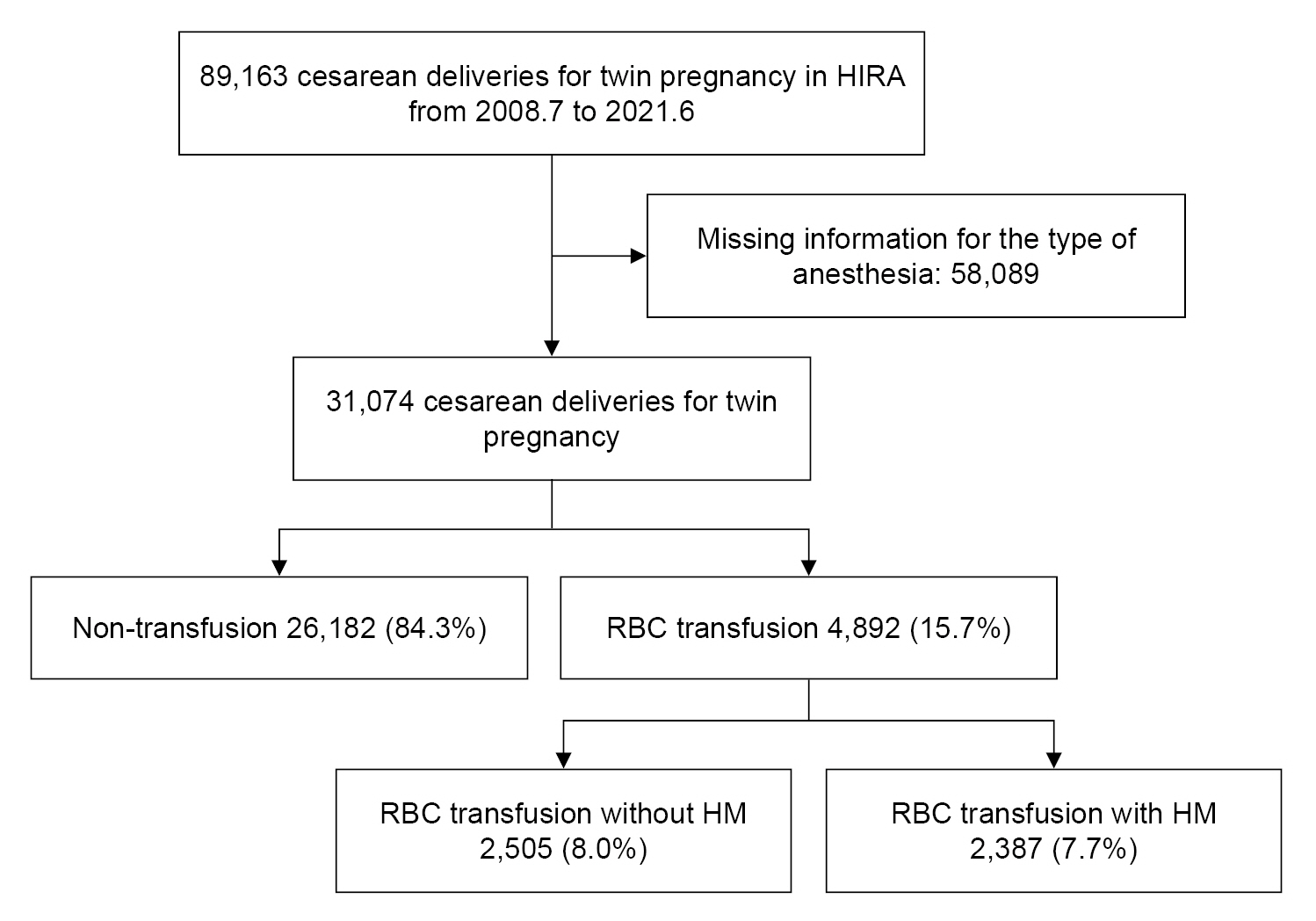
The HIRA data were searched and reviewed using the codes corresponding to cesarean delivery for twin pregnancy: R4516, R4519, R4520, R5001, and R5002. In total, 89,163 cesarean deliveries were performed for twin pregnancies between July 2008 and June 2021. However, 58,089 cases that lacked details of the anesthetic method employed were excluded. This was because, since July 2013 in Korea, cesarean deliveries have been classified under the diagnosis-related group (DRG) payment system, which resulted in the unavailability of anesthesia data in many cases. However, the data on cesarean delivery anesthesia methods were available for certain cases where fees had been claimed for the service. Finally, the data from 31,074 cesarean deliveries for twin pregnancies was extracted and analyzed (
Fig. 1).
The group of patients included in the analysis for severe PPH with RBC transfusion consisted of those who underwent at least one RBC transfusion (transfusion code: X1001, X1002, X2021, X2022, X2031, X2032, X2091, X2092, X2111, X2112, X2131, and X2132). Additionally, patients who received Ōēź 3 RBC transfusions, underwent uterine artery embolization (M6644), or underwent cesarean hysterectomy (R5001, R5002) in conjunction with RBC transfusion were classified as having RBC transfusion with hemorrhagic morbidity [
6,
10]. In total, 4,892 patients who underwent cesarean delivery for twin pregnancies received RBC transfusions. This included 2,387 patients who received RBC transfusions with hemorrhagic morbidity.
The variables analyzed include: (i) age of parturients; (ii) anesthetic method (general anesthesia: L1211, L1212, L1221, spinal anesthesia: L1213, L1223, or epidural anesthesia: L1214, L1216, L1224); (iii) previous cesarean delivery (R4516); (iv) placental disorders [morbidly adherent placenta (placenta accreta, increta, or percreta)]: O43.2, placenta previa: O44, or placental abruption: O45); (v) hypertension (I10-I15, O10, O13, or O16); (vi) diabetes mellitus (E10-E14 or O24.0-24.3); (vii) preeclampsia (O11, O14.0, O14.1, or O14.9); (viii) eclampsia (O15); (ix) failed induction of labor (O61); and (x) hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome (O14.2).
The primary outcome was to identify the risk factors associated with RBC transfusion, which reflects severe PPH in parturients who have undergone cesarean delivery for twin pregnancies. The secondary outcome was to identify the risk factors associated with RBC transfusion with hemorrhagic morbidity among parturients who have undergone cesarean delivery for twin pregnancies.
1. Statistical analysis
R version 4.1.3 (The R Foundation, Austria) was used to perform the statistical analyses. An independent t-test was used for normally distributed variables, whereas chi-squared or FisherŌĆÖs exact tests were used for dichotomous variables. Data are displayed as mean ┬▒ standard deviation or number of parturients (%). Crude and adjusted odds ratios (ORs) and 95% confidence intervals (CI) were estimated using univariate and multivariate logistic regression analyses, respectively. These were used to assess the risk factors for severe PPH requiring RBC transfusion. P values < 0.050 were considered statistically significant.
RESULTS
The characteristics of the patients classified into the non-transfusion and RBC transfusion groups have been presented in
Table 1. The rate of RBC transfusion was 15.7% (4,892 of 31,074 patients). Anesthetic methods (general anesthesia: 42.1% vs. 62.9%, spinal anesthesia: 48.4% vs. 31.4%; epidural anesthesia: 9.5% vs. 5.7% in the non-transfusion and RBC transfusion groups, respectively) were significantly different between the two groups. The rates of placental disorders (4.7% vs. 19.0%), preeclampsia (7.0% vs. 15.0%), eclampsia (0.2% vs. 0.4%), HELLP syndrome (0.1% vs. 0.5%), and hypertension (9.1% vs. 14.0%) were significantly higher in the RBC transfusion group compared to those in the non-transfusion group.
The results of univariate and multivariate analyses for PPH cases requiring RBC transfusion are presented in
Table 2. In the multivariate analysis, placental disorders (OR, 4.50; 95% CI, 4.09-4.95), general anesthesia (2.33, 2.18-2.49), preeclampsia (2.20, 1.99-2.43), HELLP syndrome (2.12, 1.22-3.68), induction failure (1.37, 1.07-1.76), and hypertension (1.31, 1.18-1.44) predicted severe PPH during or after cesarean delivery for twin pregnancy.
In the RBC transfusion group, the incidence of hemorrhagic morbidity was 48.8% (2,387 of 4,892 patients). Anesthetic methods (general anesthesia: 62.2% vs. 63.7%, spinal anesthesia: 32.7% vs. 30.0%, epidural anesthesia: 5.1% vs. 6.3% for patients without hemorrhagic morbidity and with hemorrhagic morbidity, respectively) differed significantly between the two groups (
Table 3). In the RBC transfusion group, the rates of HELLP syndrome (0.2% vs. 0.8%), placental disorders (13.9% vs. 24.3%), preeclampsia (13.1% vs. 17.1%), and parturient age (32.5 ┬▒ 3.9 vs. 33.1 ┬▒ 4.0) were significantly higher in the subgroup with hemorrhagic morbidity compared to those in the subgroup without hemorrhagic morbidity.
The results of the univariate and multivariate analyses for hemorrhagic morbidity in the RBC transfusion group are presented in
Table 4. In the multivariate analysis, the presence of HELLP syndrome (OR, 3.66; 95% CI, 1.35-9.89), placental disorders (1.96, 1.69-2.28), epidural anesthesia (1.43, 1.10-1.86), preeclampsia (1.37, 1.16-1.61), and maternal ages between 35 and 39 years (1.28, 1.12-1.46) predicted hemorrhagic morbidity in the RBC transfusion group.
DISCUSSION
The findings from this study indicate that placental disorders, general anesthesia, preeclampsia, HELLP syndrome, induction failure, and hypertension increased the adjusted ORs for PPH during or after cesarean delivery for twin pregnancy. In addition, HELLP syndrome, placental disorders, epidural anesthesia, preeclampsia, and maternal ages between 35 and 39 years can predict hemorrhagic morbidity in the RBC transfusion group. This retrospective study is the first to investigate the risk factors associated with severe PPH during or after cesarean delivery for twin pregnancy. It is also the first study to analyze the risk factors associated with hemorrhagic morbidity in the RBC transfusion group using medical data obtained from a national cohort in Korea. Our results can help obstetric and anesthesiologist teams in improving the planning and preparation for cesarean delivery for twin pregnancies.
A retrospective study on the PPH risk associated with twin gestation reported that nulliparity, diabetes, intrapartum magnesium sulfate, anemia, thrombocytopenia, and general anesthesia were significant risk factors [
6]. Among these factors, the risk associated with general anesthesia is consistent with our study findings. According to data from a 2008-2013 national cohort study conducted in Korea [
11], 3% of the study population (9,955 patients) had twin pregnancies. The factors that increased the risk of PPH after cesarean delivery were placental disorders, hypertension, and general anesthesia. These results are consistent with those of our study.
In our study, 89,163 cases of twin cesarean deliveries performed between 2008 and 2021 were identified. After excluding cases lacking information regarding the anesthetic method, 31,074 cesarean deliveries were finally analyzed. Previous studies have investigated the risk factors for cesarean delivery and PPH in twin pregnancies, which have been on the rise [
3,
4,
11]. According to a previous study, the peripartum transfusion risk factors differed between singleton and twin pregnancies. The risk factors associated with singleton pregnancies were preterm delivery and placental disorders. In comparison, the risk factors associated with twin pregnancies were gestational age Ōēź 41 weeks and hypertensive disorders [
8]. The risk factors for severe PPH observed in the present study were similar to those reported in previous studies investigating cesarean deliveries in large and small proportions of singleton and twin pregnancies, respectively [
3,
4,
11,
12]. The present study demonstrated that placental disorders, including morbidly adherent placenta (placenta accreta, increta, or percreta), placenta previa, and placental abruption, increased the risk of severe PPH requiring RBC transfusion. These findings are consistent with those of previous studies [
3,
4,
11,
13,
14], highlighting the role of placental disorders in increasing the risk of severe PPH. Notably, among the variables examined, placental disorders exhibited the highest ORs, underscoring their significant association with severe PPH. Abnormal placentation, a well-established etiology of PPH [
12,
15], was further supported by our study, thereby providing additional evidence for its contribution to adverse maternal outcomes.
Animal studies have demonstrated that volatile anesthetics can cause significant uterine relaxation, leading to uterine atony in a concentration-dependent manner. As a result, regional anesthesia may be preferable over general anesthesia in patients with placenta previa, as the use of inhaled anesthetics in general anesthesia can cause uterine relaxation, thereby leading to increased blood loss and a corresponding higher requirement for blood transfusion [
16]. However, studies suggest that administering 0.5 minimum alveolar concentration (MAC) of both desflurane and sevoflurane and 1 MAC of desflurane might be safe when administered with oxytocin during cesarean delivery [
17]. In addition, there are concerns regarding the use of neuraxial anesthesia in parturients at risk of PPH. Even when the anesthetic method is converted to general anesthesia, neuraxial anesthesia can still pose challenges, such as hypotension, which can be difficult to manage and may lead to sympathectomy, pulmonary aspiration, and airway edema [
18]. Consequently, anesthesiologists may favor general anesthesia in patients who are at risk for PPH. Indeed, conversion to general anesthesia generally occurs when the patient is experiencing bleeding or requires extensive surgery [
19]. Therefore, the choice of anesthetic method should be based on the clinical condition of the patient and the available resources within the institution [
20]. General anesthesia is employed for various indications, therefore, it is challenging to determine whether it directly caused or heightened the risk of PPH in the present study. This uncertainty arises because patients predicted to be at risk of PPH are more likely to receive general anesthesia. As a result, the presence of confounding factors cannot be entirely ruled out.
Previous studies have consistently identified maternal hypertensive disorders as independent risk factors for PPH [
13,
21,
22]. Compared to normal pregnancies, pregnancies complicated by preeclampsia are characterized by increased systemic vascular resistance, lower cardiac output, and hypovolemia [
23]. Consequently, dehydrated parturients are particularly vulnerable to the hemodynamic instability caused by PPH. In addition, decreased platelet count and hypertension can exacerbate hemorrhage and necessitate an increased need for transfusion. Preeclampsia is also associated with placental ischemia, which leads to reduced placental growth factor levels. This, in turn, leads to increased coagulopathy resulting from the activation of the fibrinolytic system [
24]. The findings of our study align with these observations, as they reveal that preeclampsia, HELLP syndrome, and hypertension are independent risk factors for severe PPH requiring RBC transfusion.
Chorioamnionitis and oxytocin exposure can help explain the development of atonic PPH after labor induction and augmentation [
25,
26]. In the present study, there was a lack of information concerning the dosage or duration of oxytocin administration for labor induction, as well as whether cesarean delivery was performed when labor was suspected based on the diagnostic code of ŌĆśfailed induction of laborŌĆÖ (O61). Despite the absence of detailed information, these findings align with previous studies that have reported a similar relationship between failed induction and increased PPH risk [
3,
9].
The risk factors associated with hemorrhagic morbidity in the RBC transfusion group were similar to those for severe PPH, including HELLP syndrome, placental disorders, and preeclampsia. However, epidural anesthesia increased hemorrhagic morbidity in the RBC transfusion group. A recent national cohort study investigating general anesthesia and epidural anesthesia also reported an increased risk of PPH [
11]. Notably, the definition of PPH used in that study may differ from the specific hemorrhagic morbidity assessed in our study. In emergency situations where a functioning epidural catheter is already in place for labor pain control, epidural anesthesia may contribute to the higher incidence of hemorrhagic morbidity. However, in our study on twin cesarean deliveries, the placement of an epidural catheter for labor pain control would be less common, as elective cesarean delivery is often planned for twin pregnancies instead of vaginal delivery. Although our study was based on HIRA claims data rather than medical records, we hypothesize that epidural anesthesia might be preferred over spinal anesthesia in cases of hemodynamic instability where acute sympathetic blockade from spinal anesthesia could complicate bleeding management. Additionally, maternal age between 35 and 39 years was also associated with hemorrhagic morbidity in the RBC transfusion group. Previous studies have presented conflicting findings regarding the impact of age on the risk of hemorrhagic morbidity, with some reporting increased morbidity in parturients older than 45 years [
27] and others in those younger than 18 years [
28]. Therefore, further research is needed to better understand the relationship between maternal age and hemorrhagic morbidity.
This study has some limitations. Firstly, the study design relied on the retrospective analysis of claims data from DRG payments obtained from the national health insurance system. As a result, several detailed clinical factors, such as uterotonic use, magnesium use, parity, number of fetuses, gestational age, chorioamnionitis, myoma, hemoglobin level, and coagulation state, could not be considered. Additionally, this study did not differentiate between elective and emergency cesarean deliveries, nor did it specify whether the parturient was in labor or prelabor during emergency procedures. These factors could have influenced the study's findings. Furthermore, the data used in this study did not allow for the assessment of temporal associations between general anesthesia and severe PPH.
In conclusion, this nationwide dataset analysis provides evidence that placental disorders, hypertensive disorders (including preeclampsia and HELLP syndrome), and induction failure are associated with an increased risk of severe PPH requiring RBC transfusion during or after cesarean delivery in twin pregnancy.