Current clinical application of dantrolene sodium
Article information
Abstract
Dantrolene sodium (DS) was first introduced as an oral antispasmodic drug. However, in 1975, DS was demonstrated to be effective for managing malignant hyperthermia (MH) and was adopted as the primary therapeutic drug after intravenous administration. However, it is difficult to administer DS intravenously to manage MH. MH is life-threatening, pharmacogenomically related, and induced by depolarizing neuromuscular blocking agents or inhalational anesthetics. All anesthesiologists should know the pharmacology of DS. DS suppresses Ca2+ release from ryanodine receptors (RyRs). RyRs are expressed in various tissues, although their distribution differs among subtypes. The anatomical and physiological functions of RyRs have also been demonstrated as effective therapeutic drugs for cardiac arrhythmias, Alzheimer’s disease, and other RyR-related diseases. Recently, a new formulation was introduced that enhanced the hydrophilicity of the lipophilic DS. The authors summarize the pharmacological properties of DS and comment on its indications, contraindications, adverse effects, and interactions with other drugs by reviewing reference articles.
INTRODUCTION
Discovered by Denborough and Lovell [1] in 1960, malignant hyperthermia (MH) is a rare autosomal dominant, pharmacogenetic, life-threatening syndrome characterized by mutations in the sarcoplasmic reticulum Ca2+ release channel in skeletal muscle cells [2,3-7]. MH can be triggered by depolarizing muscle relaxants, such as succinylcholine, and volatile anesthetics, such as halothane, enflurane, isoflurane, sevoflurane, and desflurane, which may lead to a fatal hypermetabolic state [1,3,8]. Clinical signs and symptoms vary from mild to potentially lethal and include tachycardia, hypercapnia, hypoxemia, muscle rigidity, hyperthermia, and metabolic acidosis. MH incidence is between 1:10,000 and 1:250,000; however, it affects all ethnic groups worldwide [4-6].
Dantrolene sodium (DS), which was initially introduced as an intracellular skeletal muscle relaxant, acts pharmacologically as a skeletal muscle contraction antagonist or ryanodine receptor (RyR) antagonist. DS has been clinically used since the 1980s for treating MH [4,5] and more recently for neuroleptic malignant syndrome [6], spasticity [7,8], heat stroke [9], and ecstasy intoxication [10].
DS was effective in treating porcine stress syndrome in an in vivo animal study [11], after which the United States Food and Drug Administration (USFDA) approved DS for treating human MH [12-14]. The mortality rate of MH decreased from 70–80% in 1970’s to less than 10% [6,7]. However, DS takes too long time to prepare in the clinical setting because of its chemical properties. To compensate for this drawback, new DS preparations such as azomolene, Revonto®, and Ryanodex® were introduced [15].
Most anesthesiologists who are first-liners in managing MH recognize DS as the first-line drug for treatment. However, MH is a very rare disease and DS is very expensive as well as a short life span, therefore, it is not prepared in the emergency cart of all hospitals worldwide [12,13]. There are few opportunities for anesthesiologists to experience using DS for MH. The authors summarized the pharmacological properties of DS and its derivatives as well as commented on the indications, contraindications, and interactions with other drugs.
DS AND DERIVATIVES
Over the past two decades, N-acyl hydrazone (NAH) cores were identified in numerous compounds as one of the most common functional groups in medicinal chemistry, acting on many different types of molecular targets [16]. DS is also an NAH-based hydantoin derivative [17].
DS
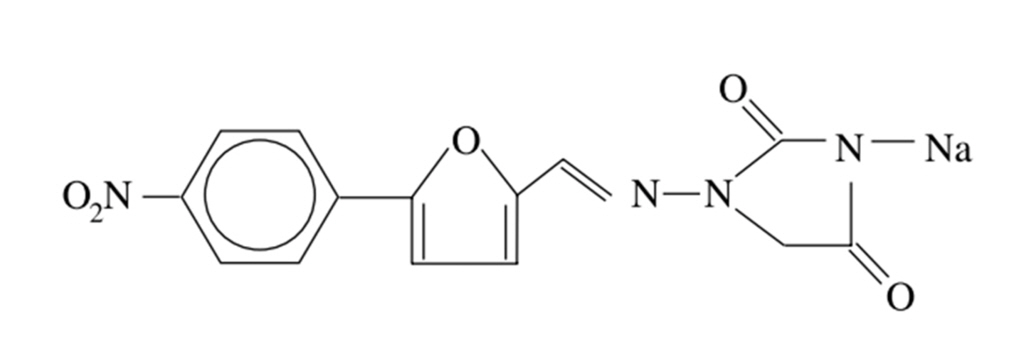
The chemical molecular formula of DS is C14H9N4NaO5. The structural formula of the hydrated salt is shown in Fig. 1 [18]. The hydrated salt contained approximately 15% water, and has a molecular weight of 399 [19].
DS is an orange-yellow crystalline powder that is poorly soluble in water. However, its slightly acidic nature somewhat increases its solubility in alkaline solutions. DS for intravenous injection was commercially supplied in 70 ml vials containing 20 mg DS, 3,000 mg mannitol, and sufficient sodium hydroxide to produce a pH of approximately 9.5 when reconstituted in pure sterile water for injection (Table 1) [20].
Revonto®
The chemical molecular formula of Revonto® (US WorldMeds) is C14H10N4O5 (Fig. 2), which can result in more rapid solubility than DS using tert-butyl alcohol. Revonto® has a half life of 36 months, is readily administered in 20 seconds [21], and is a sterile, non-pyrogenic, lyophilized formulation of DS for injection. Revonto is available in 65 ml vials containing 20 mg DS, 3,000 mg mannitol, and sufficient sodium hydroxide to yield a pH of approximately 9.5 when reconstituted with 60 ml pure sterile water for injection (Table 1) [20].
Ryanodex®
Ryanodex® (Eagle Pharmaceuticals), is an intravenous nanocrystalline suspension of DS, and is a hydrate of 1-[[[5-(4nitrophenyl)-2-furanyl]methylene]amino]-2,4-imidazolidinedione sodium salt (Fig. 2) [22]. Although Revonto® and Ryanodex® have different chemical mixtures, they both have the same chemical structures. Each Ryanodex® vial contains DS 250 mg lyophilized powder that can be rapidly reconstituted as a uniform nanoparticle suspension (less than 1 min) using only 5 ml pure sterile water for injection. This yields a suspension with a pH of approximately 10.3. Ryanodex® contains less mannitol (125 mg mannitol in a single vial) than other formulas and requires additional doses of mannitol to maintain renal function [22].
Ryanodex® is 150 times more concentrated (50 mg/ml) than regular DS (0.33 mg/ml) [23]. Schutte et al. [22,24] presented data from an MH-susceptible swine model comparing Ryanodex® with regular intravenous DS. They demonstrated that the time needed to prepare the Ryanodex® for intravenous administration was about 17 times shorter than for regular DS and therapeutic effectiveness was comparable to that of regular DS intravenous [7,22,24]. However, Ryanodex® is relatively expensive and has a short expiration date, which may limit its applicability (Table 1) [23,24].
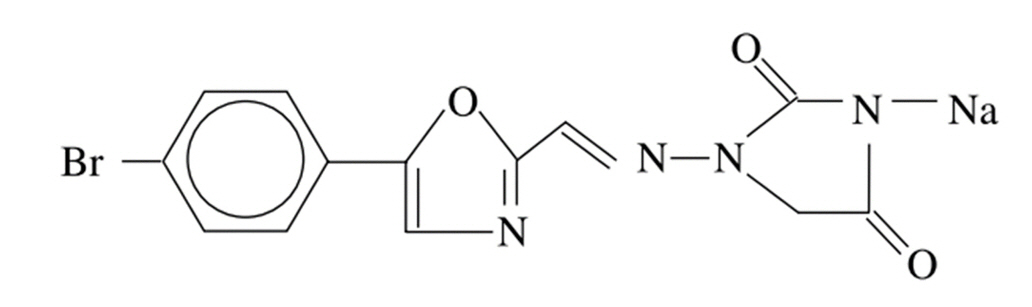
Azumolene
Azumolene(1-[[[5-(4-bromophenyl)-2-oxazolyl]methylene]amino]-2,4-imidazolidinedione, mono-sodium salt, CAS Number 105336-14-9, Molecular Formula C13H8BrN4O3 • Na) is a crystalline solid with a Formula Weight of 371.1. Azumolene must be stored at -20°C and remains stable for ≥ 2 years [18,25]. Azumolene is an analog of dantrolene and was synthesized by replacing the para-nitrophenyl group in DS with a para-bromo-phenyl group (Fig. 3). These chemical changes increase water solubility [26]. Azumolene is equipotent to DS in treating and preventing the clinical manifestations of an MH crisis secondary to inhalational anesthetics or depolarizing muscle relaxants in MH-susceptible patients. The main advantage of azumolene is its high water solubility, as it is approximately 30-fold more water-soluble than DS [27].
PHARMACOLOGIC PROPERTIES
Pharmacodynamics
DS was originally reported to inhibit the excitation-contraction coupling of skeletal muscles [11,28]. RyRs are high-conductance L-type Ca2+ channels that release Ca2+ from intracellular stores, such as the endo/sarcoplasmic reticulum (ER/SR) [29,30]. RyRs are ubiquitous in all cell types and involved in various cellular processes (E-C coupling, neurotransmission, and secretion etc.) [31]. There are three known subtypes of RYRs in mammals classified according to the initially identified tissue: skeletal-type (RyR1) is the dominant isoform in skeletal muscle, commonly referred to as skeletal ryanodine receptor; cardiac-type (RyR2) is found in the heart muscle, also known as cardiac ryanodine receptor; and brain-type (RyR3) is expressed at low levels in several tissues, but it is particularly associated with the diaphragm and brain [31-33]. For these subtypes, DS suppresses Ca2+ release from RyR1 and RyR3 [31,32]. Therefore, DS shows beneficial effects not only on MH but also on various pathologies caused by the breakdown of calcium homeostasis (e.g., stroke, ischemia/reperfusion injury, and neurodegenerative diseases) [33,34]. DS acts directly on RYR1 and RYR3 to reduce channel activation by CaM, thereby decreasing the Ca2+sensitivity of channel activation [35,36].
Pharmacokinetics
Following ingestion by mouth, approximately 70% of DS is absorbed, with peak plasma concentration being reached within 6 h. In a study where the USFDA recommended regimen for spasticity was administered orally before surgery to MH-susceptible patients, preoperative oral DS maintained the protective plasma level (> 2.8 µg/ml) for 6–18 h after induction of anesthesia, and the elimination half-life was 15.8 ± 6.0 h [37].
After intravenous administration of DS to conscious patients, the plateau maximal depression of muscle twitch response (75% depression) and the maximal depression of grip strength (42% depression) coincided with the administration of a cumulative doses of 2.2–2.4 mg/kg. This achieves a blood DS concentration of 4.2 μg/ml. Thereafter, the elimination half-life is 12.1 h, although blood concentration is maintained at a steady value within the therapeutic range for about 5 h. Residual DS concentration in the blood at 24 h after such a dose is 1.7 μg/ml and this is reflected subjectively by patients, in a feeling of weakness. This may persist for up to 48–50 h, during which time the residual blood concentration of DS decreases to 0.3 μg/ml [38].
ADMINISTRATION AND DOSAGE
DS is available as an intravenous injection and oral administration. Oral DS is much less expensive than an intravenous preparation and is usually administered for treating spasticity. It does not carry the risks of thrombophlebitis or tissue necrosis [39].
Management of MH crisis
① Intravenous DS should be administered by continuous rapid intravenous push beginning at a minimum dose of 1–2 mg/kg, with 15 min interval and continuing until symptoms subside or the maximum cumulative dose of 10 mg/kg is reached. If symptoms do not improve despite administering 7–10 mg/kg, a differential diagnosis should be made rather than additional DS administration [40-42].
The prepared DS solution should be protected from light and stored at 15–25℃, and once prepared, should be used within 6 h. The resulting alkaline solution (pH 9.5) is highly irritating to the peripheral veins and should therefore be injected into a large vein or as a fast-running fluid infusion [40-42].
If physiological and metabolic abnormalities recurred, the treatment regimen was repeated. Intravenous DS administration should be continued until symptoms subside. The effective dose to reverse the crisis is directly dependent on the individual’s degree of susceptibility to MH, amount and time of exposure to the triggering agent, and time elapsed between the onset of the crisis and initiation of treatment (Table 1). The dose of intravenous DS administered to pediatric patients was the same as that administered to adults [4,43,44].
② Ryanodex® is simplified and rapidly reconstituted to prepare a single vial within 10 s [1,4]. The time to administer a 2.5 mg/kg loading dose of Ryanodex® for a 100 kg patient is 1 min compared to > 22 min for other approved formulations [22,45]. Ryanodex® requires fewer vials (depending on the patient) and less sterile water for injection than other DS formulations [5-7]. Each vial of Ryanodex® contains 250 mg DS, and the same amount of DS as 12.5 vials of other approved formulations. Ryanodex® requires reconstitution with only 5 ml of sterile water for injection vs. 60 ml per vial for other formulations (Table 1) [5-7,22]. Ryanodex® was approved by the USFDA in 2014 [1,22,45].
Prevention of recurrence after MH crisis
Post-Crisis Follow-Up: DS capsules, 4–8 mg/kg/day, in 4 divided doses should be administered for 1 to 3 days following an MH crisis to prevent the recurrence of MH. Intravenous DS may be used postoperatively to prevent or attenuate the recurrence of MH when oral DS is not practical. The intravenous dose of DS in the postoperative period must be individualized, starting with 1 mg/kg or higher, as the clinical situation dictates [20,41,42,45].
Prophylaxis of MH before Anesthesia
There are no specific regimens for preoperative oral or intravenous DS to prevent MH. For preoperative oral DS prophylaxis, it is very difficult to maintain an effective DS blood concentration to prevent MH during the perioperative period in each patient. Moreover, a failure of oral DS therapy to prevent MH in humans has been reported previously [37,38]. Currently, prophylactic intravenous DS as well as oral DS is no longer recommended. This is based on the likelihood of adverse effects with DS prophylaxis, such as muscle weakness, hepatotoxicity, and drowsiness, and the availability of DS and appropriate patient management during MH [18]. Anesthesiologists should be aware that known triggering agents must be avoided even when DS is prepared in the emergency cart [37,38].
Preparation for intravenous injection
Each vial of intravenous DS, or Revonto®, should be reconstituted by adding 60 ml of sterile pure water for injection USP (without a bacteriostatic agent), and the vial shaken until the solution is clear. Ryanodex® is reconstituted with 5 ml. Dextrose 0.9% sodium chloride, and other acidic solutions are not compatible with intravenous DS, Revonto®, and Ryanodex® [19-22,26]. Vial contents must be protected from direct light and used within 6 h of reconstitution. Reconstituted solutions should be stored between 15 to 30°C [40-42].
Management of overdosage
Symptoms that may occur in cases of overdose include, but are not limited to, muscular weakness and alterations in consciousness (e.g., lethargy, coma), vomiting, diarrhea, and crystalluria. General supportive measures should be used to prevent acute overdose. Large quantities of intravenous fluid should be administered to avoid crystalluria. An adequate airway should be maintained and artificial resuscitation equipment should be used. Electrocardiographic monitoring should be instituted and the patient should be carefully monitored. The value of dialysis in DS overdose is currently not known [40-42].
Recommendations
As DS is not readily available in many hospitals worldwide, anesthesiologists should prepare for early diagnosis with close monitoring. Prompt effective therapies are crucial for patients with MH to survive with an initial dose of DS [3,4,44]. DS vials may be safely stored at the initial dose (e.g., 60–70 kg x 2.5 mg/kg = 150–175 mg, 8–9 vials of 20 mg DS), and the remaining DS should be immediately obtained from other centers while the initial dose was being administered [12-14]. Fig. 4 schematically illustrates the treatment algorithm for MH [3,4].
DRUG INTERACTION
DS interacts with many other medications, such as cardiac or antiarrhythmic agents (e.g., Ca2+ channel blockers), opioids, hypnotics, neuromuscular blockers, and medications for anxiety or seizures. Interactions between DS and these drugs can create serious problems; therefore, it is necessary to check them before administration.
Non-depolarizing neuromuscular blocking agents
DS has muscle relaxation properties and can potentiate non-depolarizing neuromuscular blocking agents (such as rocuronium, vecuronium, and cisatracurium). Anesthetic providers should recognize this interaction and monitor neuromuscular blockade using appropriate monitoring devices. Moreover, anesthetic providers should keep in mind that there are no antagonists for DS-induced muscle relaxation [46,47].
Ca2+ channel blockers
In an in vivo study, the interaction between verapamil, a Ca2+ channel blocker, and DS resulted in hyperkalemia and cardiovascular collapse [48,49]. DS causes cardiac arrest, atrioventricular block, acute heart failure, circulatory collapse, and ventricular fibrillation in patients with coronary artery disease treated with verapamil [50,51]. However, no such complications occurred when nifedipine was used instead of verapamil. Therefore, verapamil should be changed to nifedipine in cardiovascular patients susceptible to MH who were given verapamil and diltiazem before anesthesia [50]. The risk of serious cardiac disturbances associated with combining amlodipine and DS appears insignificant, even when amlodipine is administered at very high doses of 0.4 mg/kg [52].
Theophylline
DS and theophylline have pronounced effects on several muscle systems. Regular doses of DS (2 or 4 mg/kg) increase the theophylline lethality. This may have resulted from the synergistic action on the heart or blood vessels. In contrast, low-dose DS decreased the incidence of theophylline-induced seizure and death. This may be due to the effect of DS on Ca2+ release in skeletal muscles. The dose of DS should be decreased when used with theophylline [53].
Benzodiazepine
DS and benzodiazepines are among the available antispasmodic agents indicated for cerebral palsy, spasticity and associated pain. One comparative study observed the efficacy of DS compared with that of diazepam in children with cerebral palsy. In a double-blind study, there was no significant difference in efficacy between the two drug groups, and the combination of both drugs was more effective than each drug alone [54].
ADVERSE EFFECTS
The adverse effects of DS include dizziness; drowsiness; weakness; hives; swelling of the face, lips, tongue, throat; jaundice; difficulty breathing; chest pain; ongoing vomiting; diarrhea or constipation; problems with vision or speech; headedness; and seizures [55].
However, serious adverse effects of DS are very rare when DS is administered for a short time. The severity and onset of side effects differ according to the patient’s condition, total amount of DS, and administration route and time [56]. The North American Malignant Hyperthermia Registry reported that the incidence of adverse effects associated with DS was 35.1%, including, gastrointestinal upset, muscle weakness, excessive secretion, hyperkalemia, renal failure, and interactions with verapamil [55,56]. Of these, more than two adverse effects occurred simultaneously in 10.1% of patients. The factors responsible for the adverse effects were total dose of DS, patient age and body weight, amount of fluid administered, and severity of underlying medical conditions. Furosemide administration reduces the adverse effects of DS. The severity of the MH events did not affect the likelihood of DS-related complications [56,57].
Cardiovascular and Respiratory systems
Cardiopulmonary depression was not observed at any degree of DS-induced paralysis. At the maximum relaxant doses, DS did not produce cardiopulmonary depression in anesthetized dogs or unanesthetized sheep [58-60]. Second-degree atrioventricular block occurred at 1 and 5 weeks after MH. Decreased heart rate during sleep time was recorded using 24 h Holter monitoring [61].
Intravenous or oral administration of DS in healthy volunteers results in skeletal muscle weakness, dyspnea, respiratory muscle weakness, decreased inspiratory capacity, and pleural effusion [60,62,63]. In a dose-response study by Flewellen et al. [38], there was no change in peak expiratory flow rate, vital capacity, end-tidal carbon dioxide concentration, respiratory rate, mean arterial pressure, and heart rate.
Central nervous system (CNS)
DS has no effect on the CNS because it cannot penetrate the blood–brain barrier. However, dizziness, floating, light-headedness, drowsiness, feelings of inebriation, slurred speech, ataxia, and blurred vision can occur, regardless of the intravenous route or per os administration. It occurred immediately after DS administration and recovered over time, although it persisted for 48 h in some patients [31,57]. To decrease the incidence of side effects, the administered dose should be gradually increased [64].
Liver toxicity
DS causes liver toxicity as an adverse effect from mildly elevated liver enzyme levels during overt clinical hepatocellular injury [56,57]. Baseline liver function studies, including those of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and total bilirubin, are warranted prior to starting DS to rule out preexisting liver injury [65]. Medication should be stopped immediately if liver function becomes impaired [66]. The incidence of hepatic toxicity is related to the total amount of DS, duration of administration, and female sex, especially over 35 years of age as well as old age. It usually recovers completely within 1–3 months [67].
Gastrointestinal disturbances
These complications commonly occur when DS is orally administered. Anorexia, gastric irritation, abdominal cramping, constipation, dysphagia, nausea, vomiting, and diarrhea usually occurs [55-57].
Volume overload and electrolyte imbalance
Because each 20 mg vial of DS requires 60 ml of sterile water diluent, the median (1st and 3rd quartile) of co-administration of sterile water is required to be 666 (284, 1,800) ml, respectively [56,57]. The complications associated with administration increased with the dose of DS, and could be significantly increased by fluid administration and decreased by the administration of furosemide. Severe complications were most likely due to the patient’s underlying medical condition rather than DS administration. When DS is administered, clinicians should exercise vigilance for changes in intravascular fluid volume and the subsequent development of cardiorespiratory complications [55-57].
Thrombophlebitis
When DS is infused through a small peripheral vein, extravasation, thrombophlebitis, and tissue necrosis may occur. A lyophilized formulation of an alkaline solution (pH 9.5) induces an acute inflammatory reaction in the vascular endothelium. DS should be administered to large veins through a free-flowing large-bore intravenous catheter [56,57].
EFFECTS OF DS ON PREGNANCY AND FETUS
Pregnancy
DS is an FDA pregnancy category C. DS should be used during pregnancy only if its potential benefits justify the potential risks to the fetus [64,68]. The prevalence of MH susceptibility is approximately 1/125,000 in Cesarean deliveries, which is similar to the prevalence reported in non-obstetrical surgery inpatients. Previous studies have suggested that stocking DS in maternity units is justified [64,68]. In an animal study, postpartum uterine atony was reported in a woman who received prophylactic intravenous DS using oxytocin after delivery [69,70].
There have been no adequate or well-controlled studies in pregnant women. Available data from case reports of the intravenous administration of DS during pregnancy are insufficient to evaluate the drug-associated risk of major birth defects, miscarriages, or adverse maternal and fetal outcomes. DS readily crosses the placenta; however, no serious adverse events have been reported in neonates following the maternal administration of DS prior to delivery. Although an equilibrium between maternal and fetal plasma DS concentrations was apparent at 5 min, the fetal levels of DS were approximately 10% of the mother's [71-73].
Lactation
DS has been detected in human milk at concentrations of less than 2 μg/ml during repeat intravenous administration over 3 days. The estimated half-life of DS in breast milk is approximately 9 h. Based on these data, the amount of infant exposure to DS through breastfeeding would be negligible 2 days after the last maternal dose. If used in the short term, the data suggest that alternate feeding methods may be pursued during active DS treatment and breastfeeding may be restarted 1–2 days after treatment is stopped [74].
Fetus and neonate
DS is administered to mother during the cesarean section, fetal blood concentration will be 65% of maternal plasma level [61,64]. No adverse effects of DS have been detected by extensive testing of fetuses and neonates in certain reports [64,68]. In rare cases, Floppy infant syndrome has been reported in affected fetuses and neonates [57]. The use of dantrolene in MH-susceptible pregnant patients did not cause noticeable adverse effects in the fetus or neonate [64].
INDICATION OTHER THAN MH
DS can be used for the treatment of MH and other diseases related to abnormalities in L-type calcium channel receptors, such as RyRs [1-3,5-7].
Spasticity
USFDA-approved uses for DS include muscle spasticity disorders, as seen with upper motor neuron disorders, including stroke, traumatic brain injury, spinal cord injury, cerebral palsy, and multiple sclerosis [7,8]. It is the only USFDA-approved oral peripherally-acting antispasmodic medication for these disorders [75-77]. It can be administered at an initial daily dose of 25 mg, which can be increased to 100 mg 4 times a day for a maximum total dose of 400 mg per day [78].
Neuroprotection
Induced normothermia or hypothermia has become a treatment modality for reducing fever burden in neurological injury [79-84]. DS reduces the metabolic effects of fever in the presence of neurological injury by reducing shivering gain and shivering threshold [31,85,86]. Although occasionally used as an add-on to anti-shivering drug, DS usually causes less sedation and muscle relaxation than the medications commonly used to treat shivering, and, at the same time, may also be neuroprotective [87].
Ca2+ signaling is crucial for maintaining normal neuronal functions such as membrane excitability, neurotransmitter release, cellular growth, differentiation, and cell death. DS is also an effective drug for disrupting Ca2+ homeostasis, as reported in neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, and spinocerebellar ataxia [5,79,80]. Binding of DS to RyRs in the brain may protect neurons from disruptions in Ca2+ homeostasis [79-84].
Neuroleptic malignancy
DS is also used for treating drug-induced fever, such as neuroleptic malignant syndrome [6], overdose of 2,4-dinitrophenol (a banned "fat burner" medication that interrupts ATP synthesis and causes hyperthermia) [88,89], lysergic acid diethylamide (LSD), and MDMA (3,4-methylene-dioxy-meth-amphetamine, ‘Ecstasy’) toxicity [10,36,90]. It can also be used to treat the serotonin syndrome, anticholinergic poisoning, sympathomimetic poisoning [91,92].
Sepsis and toxic shock
In sepsis and toxic shock syndromes such as Staphylococcus aureus bacteremia with skeletal muscle hypermetabolism, DS may be considered early if specific antibiotic therapy alone is not successful [93].
MH is triggered by halogenated inhalational anesthetics and viral infections. The mechanisms underlying rhabdomyolysis and fever in corona virus disease 2019 (COVID-19) may be similar to those in MH. Therefore, DS will be effective in most patients with severe COVID-19 with acute respiratory distress syndrome and lymphopenia as well as disorders of the central or peripheral nervous system, cardiac arrhythmias, cardiomyopathy, rhabdomyolysis, coagulopathy, and shock [94-96].
Classic heat stroke and emotional heat stroke (EHS)
Heat stroke is usually diagnosed when core temperature exceeds 40.6℃ [97]. The symptoms of heat stroke are similar to those of MH [97,98]. As a rapid decrease in body temperature is important for managing heat stroke, DS has been used in conjunction with various physical cooling techniques [10,99]. Individuals genetically susceptible to MH, with a positive MH response in the in vitro contracture test, may be at an increased risk of exertional heat illness and exertional rhabdomyolysis [100]. One well-known case reported was that of a child who had an unequivocal MH episode during anesthesia and later died of EHS [100]. DS antagonizes RyRs within the SR, inhibiting Ca2+ release into the cytosol and reversing muscle rigidity as well as body heat production. However, DS did not reduce cooling time, multiple organ injury, or length of hospital stay in patients with classic heat stroke and EHS in several studies [97-100].
Cardiac arrhythmias
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is one of the most malignant genetic arrhythmogenic disorders. It manifests as exercise- and/or stress-induced premature ventricular complexes, polymorphic or bidirectional ventricular tachycardia, or sudden death and is usually associated with vigorous physical exercise or mental stress [101,102]. The most common CPVT subtype, type 1, is a dominantly inherited disease caused by mutations in the cardiac RyR2 gene [103]. Mutations in RyR2 cause increased Ca2+ sensitivity, which can lead to spontaneous Ca2+ release from the SR, generation after depolarization, and triggered activity. Intravenously administered DS suppresses ventricular arrhythmias in congenital RyR2 defect [103]. DS corrects defective interdomain interactions within RyR2 in failing hearts and CPVT, inhibits spontaneous Ca2+ leakage, and improves cardiomyocyte function. Thus, DS has the potential to treat heart failure and CPVT by specifically targeting RyR2 [101-104]. DS is safe and clinically effective for treating cardioglycoside poisoning [105].
CONTRAINDICATION AND WARNING
There are no contraindications to using intravenous DS for treating of MH. However, caution should be exercised when administering DS to patients with hypersensitivity, impaired hepatic function, liver cirrhosis, non-alcoholic steatohepatitis, and hepatitis B or C [66,106].
According to the manufacturer’s manual, Pharmaceutical, Inc., and USFDA, DS should be used with particular caution in females and in patients over 35 years old, in view of the apparent greater likelihood of drug-induced, potentially fatal hepatocellular disease in these groups. Other reports suggested a higher proportion of hepatic events with fatal outcomes in elderly patients undergoing DS. However, most of these cases were complicated by confounding factors such as intercurrent illnesses and/or concomitant potentially hepatotoxic medications. In general, dose selection for elderly patients should be performed with caution, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, concomitant disease, or other drug therapy. Similar to all patients undergoing DS, elderly should patients receive the lowest dose compatible with an optimal response [19,67,107,108].
CONCLUSION
In the field of Anesthesiology, DS is the drug of choice in the emergency care setting for treating MH crisis. Additional personnel and efforts are required to assist in the preparation process. Therefore, it is necessary to understand the pharmacological properties of DS so that it can be used according to its indications and adverse effects.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Hong Seuk Yang, Tae-yun Sung, Yong Beom Kim. Formal analysis: Junyong In, Tae-yun Sung. Project administration: Hong Seuk Yang. Visualization: Junyong In, Yong Beom Kim, Shofina Sultana. Writing - original draft: Hong Seuk Yang, Tae-yun Sung. Writing - review & editing: Hong Seuk Yang, Jae Moon Choi, Junyong In, Tae-yun Sung, Yong Beom Kim, Shofina Sultana. Resources: Hong Seuk Yang, Jae Moon Choi. Supervision: Hong Seuk Yang, Jae Moon Choi, Tae-yun Sung, Yong Beom Kim, Shofina Sultana. Validation: Shofina Sultana.