Single and double injection paravertebral block comparison in reduction mammaplasty cases: a randomized controlled study
Article information
Abstract
Background
This study compares the analgesic effects and dermatomal blockade distributions of single and double injection bilateral thoracic paravertebral block (TPVB) techniques in patients undergoing reduction mammaplasty.
Methods
After obtaining ethics committee approval, 60 patients scheduled for bilateral reduction mammaplasty were included in the study. Preoperatively, the patients received one of single (Group S: T3–T4) or double (Group D: T2–T3 & T4–T5) injection bilateral TPVBs using bupivacaine 0.375% 20 ml per side. All patients were operated under general anesthesia. The T3–T6 dermatomal blockade distributions on the midclavicular line were followed by pin-prick test for 30 min preoperatively and 48 h postoperatively. All patients received paracetamol 1 g when numeric rating scale (NRS) pain score was ≥ 4, and also tramadol 1 mg/kg when NRS was ≥ 4 again after 1 h. The primary endpoint was NRS pain scores at postoperative 12th h. The secondary endpoints were dermatomal blockade distributions and NRS scores through the postoperative first 48 h, time until first pain and the analgesic consumption on days 1 and 2.
Results
Fifty-two patients completed the study. The NRS pain scores at 12th h were similar (right side: P = 0.100, left side: P = 0.096). The remaining NRS scores and other parameters were also comparable within the groups (P ≥ 0.05). Only single injection TPVB application time was shorter (P < 0.001).
Conclusions
The single injection TPVB technique provided sufficient dermatomal distribution and analgesic efficacy with the advantages of being faster and less invasive.
INTRODUCTION
According to the 2013 US national survey, approximately 86% of patients experience pain after surgery. Seventy-five percent of these have acute, moderate or extreme immediate pain with 74% having severe pain after discharge [1].
In United States, 520.000/1.8 million cosmetic surgical procedures in 2018 were related to breast, and approximately 43.600 of them have aesthetic breast reduction surgery [2]. Previously; it was reported that 28% of reduction mammaplasty patients experience acute pain after the operation, 20% had chronic constant pain at least once per week for longer than 3 months, and 7% had moderate to severe pain [3]. Another study showed 22% of bilateral reduction mammaplasty patients with postoperative acute pain still experience chronic pain after a year [4].
Even with advanced surgical techniques and pain management strategies, recent studies have continued to notify similar or elevated chronic pain incidences based upon the surgical procedure types [3-7]. The reason is probably the complex innervation of the anterior chest wall and the breast area. Brachial plexus branches such as long thoracic and thoracodorsal nerves lie laterally and innervate the serratus anterior and latissimus dorsi muscles. In addition, superiorly located lateral and medial pectoral nerves innervate pectoralis major and minor muscles. Ventral branches of spinal nerves travel from posterior to lateral as intercostal nerves, give their lateral cutaneous branches at the anterior or mid-axillary line and proceed as anterior cutaneous branches. These innervate the related dermatomes on their path while covering both lateral and medial sides of the breast. Intercostobrachial nerve which originates from T2 lies on the axillary region. Different combinations of these nerves can be blocked by various chest wall block strategies, and the most commonly used ones are thoracic paravertebral (TPV), interpectoral & pectoserratus, serratus anterior and erector spinae plane blocks. The optimum prevention and management of postoperative acute pain with multimodal analgesia including regional analgesia techniques is extremely important, and should be planned appropriately considering the anatomy, invasiveness of the surgery, and also the incision to improve the patient outcomes [1, 8-10].
Thoracic paravertebral blocks (TPVBs) reduce pain and systemic opioid requirement effectively, lower risk of postoperative pulmonary complications, allow catheter insertion for continuing blocks and provide multiple levels of analgesia [9]. Currently; its practice has become safer and more successful with the guidance of ultrasound (US). As the imaging of the paravertebral space (PVS), tip of the needle and distribution of the local anesthetic (LA) can all be easily obtained; it is used as the first-choice analgesia technique in especially breast cancer (mastectomy with/without lymph node removal) and also in reconstruction (implant or expander placement, pedicled latissimus dorsi flap surgery) surgeries [11-13]. With regards to the reduction mammaplasty surgeries, our reason of choosing TPVBs as regional analgesia technique is mostly about blocking the breast area dermatomal innervation to relieve the surgical pain without causing any muscle blockade which is not necessarily due to superficial nature of this surgery. During the TPVB performances, single or multiple injection techniques can be used to obtain sufficient LA and related dermatomal analgesia spread. Four or five ml LA spread per dermatome is considered to be ideal and effective, but it is impossible to manage LA spread during or after deposition. To our knowledge, the superiority of these TPVB techniques have been confounding in thoracic and breast surgeries [13,14], and scarce especially in reduction mammaplasty surgeries.
In this prospective randomized controlled study; the efficiencies of US-guided single (T3–T4 level) and double (T2–T3 and T4–T5 levels) injection TPVB techniques are compared in patients who underwent reduction mammaplasty. The primary endpoint was numerical rating scale (NRS) pain score at 12 h after surgery. Our null hypothesis was that single injection and double injection US-guided bilateral TPVB group NRS scores at 12th hour would have “no difference”. The secondary endpoints included the NRS pain scores and the dermatomal blockade distribution through the postoperative first 48 h, block application times, number of patients experienced hypotension or required fentanyl intraoperatively, length of stay in postanesthesia care unit (PACU), postoperative time until first pain (NRS ≥ 4), analgesic consumption, incidence of postoperative nausea and vomiting (PONV) and duration of sleep on postoperative days 1 and 2, and eventually patient and surgeon satisfaction scores.
MATERIALS AND METHODS
Setting and study population
After institutional ethics committee approval (Istanbul University, Istanbul Faculty of Medicine: 2016/1282); 60 patients, who were scheduled for elective bilateral reduction mammaplasty (without adjunctive liposuction to the breast) in the Department of Plastic, Reconstructive and Aesthetic Surgery between December 2016 and December 2017 and gave written informed consent, were enrolled in the study according to the following inclusion criteria: female gender, aging between 18 and 70 years, American Society of Anesthesiologists physical status of 1-3, understanding the instructions for using the NRS pain scores and replying the study-based questions, lack of contraindications to regional anesthesia (patient refusal, allergy to a LA, local infection and coagulopathy) and especially to TPVB, absence of mental/psychiatric disorders, chronic analgesic/opioid use and alcohol/illicit drug use. Patients were randomized to a single (Group S) or a double (Group D) injection TPVB groups using the sealed envelopes technique.
This prospective randomized controlled clinical study is reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement [15] and registered on ClinicalTrials.gov (NCT04517331).
Interventions
On the day of the surgery, standard American Society of Anesthesiologists monitors (electrocardiogram, non-invasive blood pressure measurement, oxygen saturation monitorization and body temperature measurement) were applied to all patients in the operating room. Peripheral intravenous vascular access and oxygen via a facemask were administered.
The patients were placed in the sitting position and spinous processes were marked from C7 to T6. After skin disinfection, a high frequency linear US probe (5-13 MHz; GE Healthcare) was placed 2–2.5 cm lateral to the midline longitudinally to define the level-related transverse processes and the pleura between them. For skin infiltration of all determined injection sites, 1 ml of lidocaine 2% was applied. By using a 22-gauge, 50-mm insulated stimulating needle (Stimuplex A; B Braun) and out-of-plane technique, the blocks were performed at the T3–T4 level bilaterally in Group S (single injection TPVB group), and at both T2–T3 and T4–T5 levels bilaterally in Group D (double injection TPVB group) patients to block the dermatomes between the T2 and T6 (breast innervation area). During the performance of all TPVBs, the needle was advanced till the PVS and LA was injected after negative aspiration while the downward displacement of the parietal pleura was observed. As an LA, 20 ml bupivacaine 0.375% was used in all patients per side (Group S: 20 ml/injection and Group D: 10 ml/injection). All TPVB procedures were performed by the same 3 senior anesthesiology residents (V.A.O, H.P, H.C.G), always under the supervision of 2 attending anesthesiologists who are experienced in both regional anesthesia and its training (E.A.S, N.S).
The bilateral TPVB application time was defined as the time-period between the needle insertion at the first determined level and the needle withdrawal from the last determined level. Then, the sensorial blockade was tested bilaterally between T3 and T6 dermatomes (4 regions) on the midclavicular line in every 5 min through the first 30 min after TPVB performances, by using the pin-prick test. The anesthesiologists who were randomized to the group of that specific patient (M.O.S, V.A.O, H.P, H.C.G, K.M.T) evaluated the sensorial blockade of dermatomes as “normal”, “decreased sensation” or “total anesthesia” separately on both sides. The answers such as “decreased sensation” or “total anesthesia” in different dermatomal regions within T3–T6 were accepted as “successfully blocked dermatome”, and the numbers of blocked dermatomes out of 4 dermatomal regions were noted for the right and the left sides. If the number of blocked dermatomes on midclavicular line of any sides was ≤ 2/4 at 30th min, the block was accepted as “failed” and the patient was excluded from the study.
All patients were operated under standard general anesthesia (induction: midazolam 2 mg, fentanyl 1–2 µg/kg, propofol 2–3 mg/kg, rocuronium 0.6 mg/kg, and maintenance: sevoflurane 2%, 50% O2-50% N2O mixture) by the same surgical team (E.K, S.K., U.E). Prophylactic 4 mg ondansetron was injected intravenously to all. The vital parameters of the patients were followed as a routine anesthesia follow-up. Intraoperative atropine (0.5 mg) or ephedrine (5 mg) was administered, if the heart rate (HR) was < 50 beats/min or mean arterial pressures (MAP) decreased > 20% below preinduction value (hypotension). If an intraoperative ≥ 20% increase above preinduction values in MAP or HR was observed, additional fentanyl (1 µg/kg) was applied. The number of patients experienced hypotension or conversely required fentanyl were recorded. At the end of the surgeries, all patients were administered paracetamol (1 g) intravenously right before extubation to contribute to the postoperative multimodal analgesia, and then extubated. The durations of surgery and general anesthesia, and also length of stay in PACU were all noted. Discharge from PACU was determined using the White Fast-tracking score ≥ 12 whereas all parameters were ≥ 1 in different categories [16].
The NRS pain scores (0: no pain, 10: worst pain imaginable) and the dermatomal blockade distribution/numbers of blocked dermatomes of all patients on both midclavicular sides were recorded immediately after anesthesia recovery and at 1, 2, 6, 12, 24 and 48 h after surgery. The postoperative time until first pain (NRS ≥ 4) was noted. Patients in both groups received intravenous paracetamol 1 g when NRS ≥ 4 (maximum dose 4 x 1 g per day), and also tramadol 1 mg/kg if NRS ≥ 4 again after 1 h (maximum dose 4 x 1 g/kg per day) in the PACU or on the wards. The rescue analgesic consumption/the numbers of paracetamol and tramadol requirements, incidences of PONV (even just a little nausea feeling) and durations of sleep on postoperative days 1 and 2, and also patient and surgeon satisfaction scores (0: very unsatisfied, 1: unsatisfied, 2: satisfied, and 3: very satisfied) were all documented.
Different anesthesiologists who did not participate in the TPVB process of that specific patient and were totally blinded to the group (M.O.S, V.A.O, H.P, H.C.G, K.M.T) collected the postoperative data.
Statistical analysis
The sample size of this study was calculated in concordance with previous studies that compared the postoperative visual analog scale (VAS) scores of the TPVB applications in thoracoscopic surgeries [13,17]. We calculated that 24 patients per group were required for a minimal NRS difference of 1, when α = 0.05, standard deviation (SD) = 1.2 and the power = 0.8. Therefore, 30 patients were assigned to each group to overcome possible dropouts.
The data were expressed as mean±SD, number and median (interquartile range). Student's t-test was used for parametric data such as age, body mass index weight of resected breast, block application time, durations of surgery and general anesthesia, length of stay in PACU. After normality test is applied, Mann-Whitney U test was used for the comparison of non-parametric data such as NRS scores, the number of blocked dermatomes, postoperative time until first pain, the numbers of paracetamol and tramadol requirements, durations of sleep on postoperative days 1 and 2, patient and surgeon satisfaction scores. In case of need; one of Pearson Chi-Square (χ2) or Fischer’s exact tests was used for categorized variables such as American Society of Anesthesiologists physical status, breast reduction incision types, breast reduction pedicle types, number of hypotensive patients, number of patients required fentanyl and incidence of PONV. A P value < 0.05 was accepted as statistically significant. SPSS Version 21.0 (IBM Co.) program was used for statistical analysis.
RESULTS
A total of 60 patients scheduled for elective reduction mammaplasty were eligible and allocated to 1 of the 2 groups equally. Two patients from each group were excluded from the study because of their “failed blocks”, and 4 patients from Group D were excluded because of missing data. Fifty-two patients completed the study: 28 in Group S and 24 in Group D (Fig. 1).
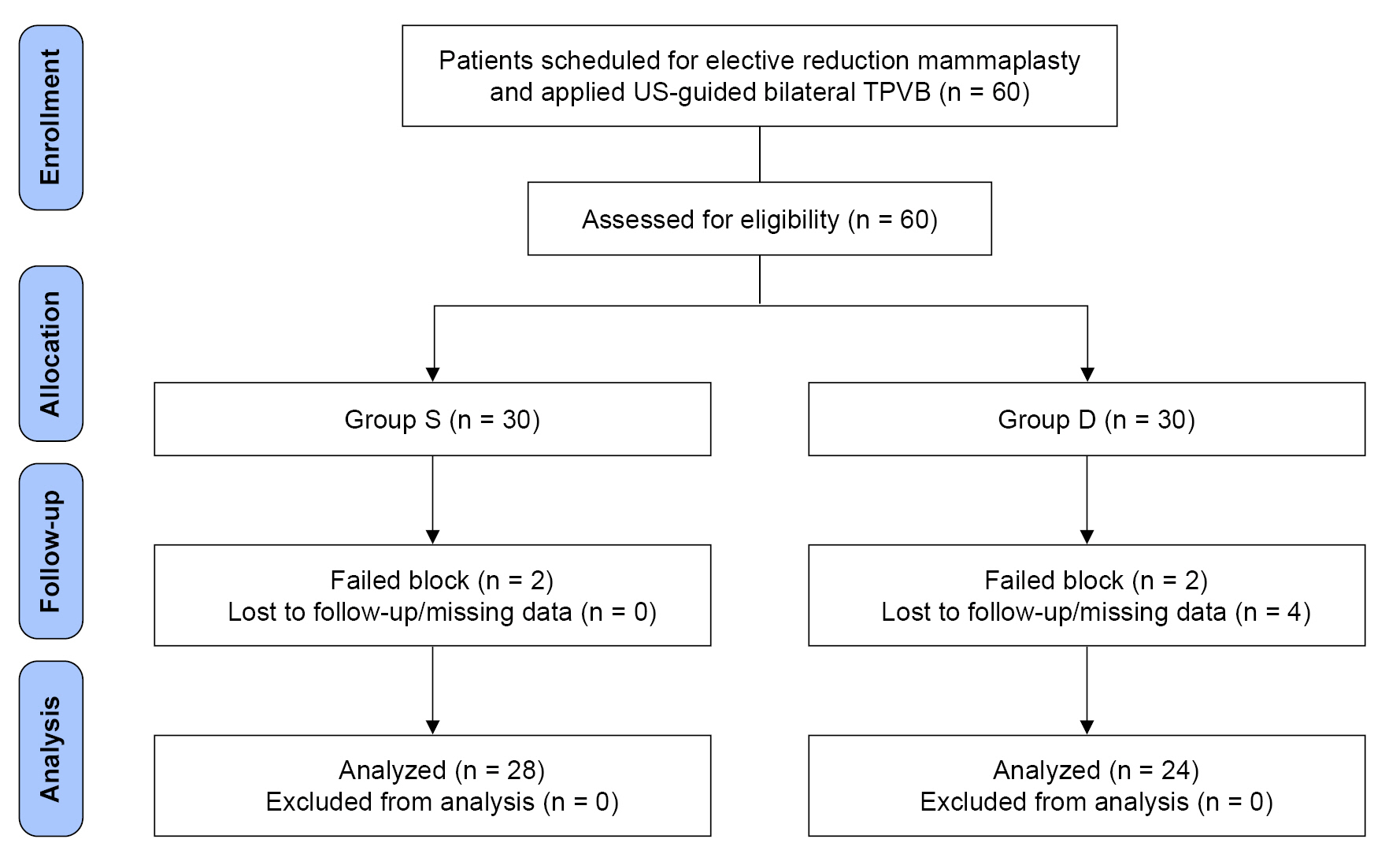
CONSORT diagram of the Groups S and D. CONSORT: consolidated standards of reporting trials, US: ultrasound, TPVB: thoracic paravertebral block. Group S: patients with single injection TPVB, Group D: patients with double injection TPVB.
Demographic details
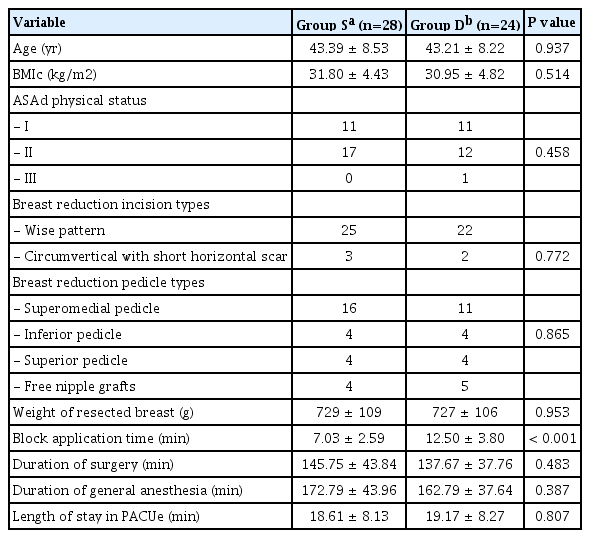
Demographic data of the patients, surgical characteristics, durations of surgery and general anesthesia, and length of stay in PACU were all similar in groups (P ≥ 0.05). The total bilateral TPVB application time was significantly longer in Group D than Group S (P < 0.001) (Table 1).
Endpoint details
There was no difference in 12th h postoperative NRS pain scores between Groups S and D (right side: P = 0.100, left side: P = 0.096). NRS pain scores at other time points were also comparable (P ≥ 0.05). At all times, the mean NRS scores were ≤ 3 (range, 0 to 5) in both groups. Only 1 patient in Group D described her NRS as “7/10” on postoperative 6th h, which did not affect the mean NRS score of the whole group, and was controlled easily by ordered analgesics (Table 2). The numbers of blocked dermatomes within 30 min after TPVB and through postoperative 48 h (Table 3) were both similar in groups (P ≥ 0.05).
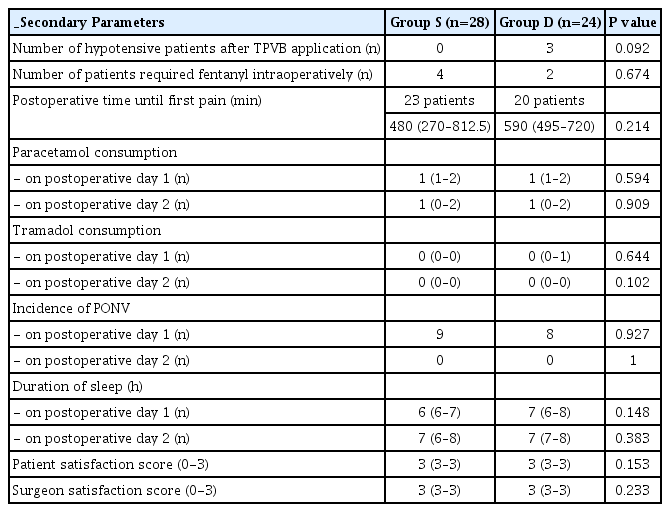
Five patients from Group S and 4 patients from Group D did not report any pain during the whole follow-up period (NRS < 4). When the postoperative time until first pain of the remaining patients was compared (Group S: 23 patients (480 [80-1,240 min]) and Group D: 20 patients (590 [120-1,400 min])), no difference was found (P = 0.214). Accordingly; the total analgesic requirements and sleep durations on postoperative days 1 and 2 were alike (P ≥ 0.05) (Table 4).
Hypotension was the only observed adverse effect in 3 patients of Group D, and responded promptly to position change and fluid therapy. Conversely, 4 patients from Group S and 2 from Group D were applied fentanyl intraoperatively. The PONV incidences on postoperative days 1 and 2 were similar in both groups, all patients experienced only nausea for a short period of time. The satisfaction scores were both high (3 [range, 2 to 3]) in groups (P ≥ 0.05) (Table 4).
None of the patients of any groups required intraoperative atropine or ephedrine with respect to hemodynamic changes. No anesthesia- or surgery-related complication occurred during the study.
DISCUSSION
In this study; US-guided single and double injection TPVB techniques performed for elective reduction mammaplasty surgeries were assessed. No superiority was determined in terms of blocked dermatome distributions between T3 and T6 levels and perioperative analgesic effects. The only different parameter of the two groups was the length of the block procedure. Double injection group had a longer block process as it would be expected.
The anesthetic/analgesic advantages and the effectiveness of TPVBs with single and multiple injection methods have been demonstrated for various reconstructive and breast cancer surgeries in previous studies [12,14,18-25]. Then, following the demonstration of their postoperative analgesic benefits, TPVBs were also advocated over general anesthesia in some studies [20-22]. However, the number of publications on this topic has been limited because of the technical challenges of the block and the complication risks [18,19,20,24]. In our institution, single injection TPVB is a routine procedure for all reduction mammaplasty patients and they benefit a lot from the analgesic effects of the technique [18,25]. Nevertheless; there have always been doubts about the single injection cranio-caudal T3-T6 distribution sufficiency and complete surgery/surgical incision area blockade for macromastia patients.
Different techniques, dyes and volumes in US-guided cadaver studies resulted in various distributions. Luyet et al. [26] observed variable distributions between T2 and T6 levels after a 10 ml of contrast solution. Cowie et al. [27] injected 20 ml of contrast solution with the transverse technique and showed 4.5 PVS distribution in single and 6 in double injections.
Dermatomal distributions of TPVB applications performed under the guidance of anatomical markings and neurostimulators were evaluated in two previous studies, and wider spread was reported in multiple injection groups [12,21]. Naja et al. [21] performed multiple injections for different surgical procedures and reported their clinical benefits. Conversely; Kaya et al. [12] could not find any clinical analgesic contribution of this wide distribution.
The dermatomal distribution of US-guided TPVB performances were also reported. Marhofer et al. [28] injected 20 ml mepivacaine 1% into bilateral T6 levels, and then observed the 3-dimensional distributions with the magnetic resonance imaging technique and evaluated the sensory blocks with the pin-prick test. In the aspect of cranio-caudal LA distribution, 4 vertebral levels on the left and 3.5 on the right side were determined. Additionally, with regards to the sensorineural dermatomal blockage, they found 9.8 vertebral levels on the left and 10.7 on the right side. Wider sensorineural distribution than LA distribution was reported. This conclusion showed that the somatic anesthetic distribution cannot be interpreted precisely. Twenty milliliters may cover 4 dermatomes; however, the spread through the epidural space, prevertebral area and other directions may raise concerns over cranio-caudal distribution and clinical efficiency. That is why; our study is not based on the total number of blocked dermatomes but focused on the sufficient dermatomal blockade of the bilateral surgical fields (T3–T6). Moreover; if the number of blocked dermatomes at any side was ≤ 2/4 at 30th min, we accepted TPVB as “failed”.
Ben-Ari et al. [29] placed bilateral US-guided PVB catheters in elective abdominal surgery patients and injected lidocaine 10 ml. In average, 5 dermatomes were blocked. Renes et al. [30] administered 20 ml of ropivacaine 0.75% from a TPVB catheter, and 6 dermatomes were blocked in average. In a recent mastectomy study published by Uppal et al. [14], US-guided single and multiple (five) injection (25 ml of 0.5% ropivacaine) TPVB techniques provided similar dermatomal distribution and analgesia duration. Their results emphasized the value of visualized needle tip under US-guidance, and the probability of a more precise and successful single injection block. In contrast; in patients undergoing total mastectomy with axillary dissection, multiple injections were reported to be superior in the distribution of LAs [13]. These showed us that US-guided TPVB studies in breast surgeries still have conflicting results and made us think whether our single injection TPVBs could be improved by multiple injections. Could LA distribution be augmented? Could more effective analgesia be enabled? Nevertheless; within the first 30 min after TPVBs and 48 h after surgeries, dermatomal blockade distribution was similar at all time points in our patients.
The NRS pain scores at all time points, time until first pain and postoperative analgesic consumptions did not show any difference between our two groups. These findings are compatible with the sufficiently blocked bilateral dermatomes of both. Accordingly, Uppal et al. [14] obtained similar intraoperative opioid needs, postoperative NRS scores and analgesia times in two groups of their unilateral mastectomy surgeries. Kaya et al. [12] also determined comparable analgesic parameters and opioid use despite a significant difference in the number of blocked dermatomes in video-assisted thoracic surgery patients. So; these clearly stress the importance of determining the certain TPVB level for the surgery and the incision, rather than obtaining a wide cranio-caudal block distribution.
Again as reported before [12,14], single injection block application times were shorter. Although this affected the patient satisfaction positively in video-assisted thoracic surgery [12], we found no difference and our patients appreciated only the effective analgesia state.
We think this study has one important shortcoming. Although chronic pain occurrence following reconstructive breast surgery is known [3,7], this study has focused on postoperative acute pain follow-ups.
In conclusion, the single injection US-guided TPVB technique provided sufficient dermatomal distribution and analgesic efficacy in patients undergoing reduction mammaplasty with the advantages of being faster, less invasive and equally efficient.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Emine Aysu Salviz, Nukhet Sivrikoz. Data curation: Vecih Anil Ozonur, Erol Kozanoglu, Soner Karaali, Huru Ceren Gokduman, Hacer Polat. Formal analysis: Emine Aysu Salviz, Hacer Polat, Mukadder Orhan-Sungur. Methodology: Vecih Anil Ozonur, Emine Aysu Salviz, Nukhet Sivrikoz. Visualization: Erol Kozanoglu, Mukadder Orhan-Sungur. Writing - original draft: Vecih Anil Ozonur, Huru Ceren Gokduman, Emine Aysu Salviz, Mukadder Orhan-Sungur. Writing - review & editing: Huru Ceren Gokduman, Emine Aysu Salviz. Investigation: Vecih Anil Ozonur, Emine Aysu Salviz. Software: Soner Karaali. Supervision: Emine Aysu Salviz, Nukhet Sivrikoz, Ufuk Emekli, Mehmet Kamil Tugrul.