Revolutionizing trauma care: Advancing coagulation management and damage control anesthesia
Article information
Abstract
Despite advances in emergency transfer systems and trauma medicine, the incidence of preventable deaths due to massive hemorrhage remains high. Recent immunological research has elucidated key mechanisms underlying trauma-induced coagulopathy in the early stages of trauma, including sympathoadrenal stimulation, shedding of the glycocalyx, and endotheliopathy. Consequently, the condition progresses to fibrinogen depletion, hyperfibrinolysis, and platelet dysfunction. Coexisting factors such as uncorrected acidosis, hypothermia, excessive crystalloid administration, and a history of anticoagulant use exacerbate coagulopathy. This study introduces damage-control anesthetic management based on recent insights into damage-control resuscitation, emphasizing the importance of rapid transport, timely bleeding control, early administration of antifibrinolytics and fibrinogen concentrates, and maintenance of calcium levels and body temperature. Additionally, this study discusses brain-protective strategies for trauma patients with brain injuries and the utilization of cartridge-based viscoelastic assays for goal-directed coagulation management in trauma settings. This comprehensive approach may provide potential insights for anesthetic management in the fast-paced field of trauma medicine.
INTRODUCTION
Despite improvements in emergency transfer systems and trauma medicine, preventable deaths due to massive hemorrhage remain a potential concern [1]. Most traumatic deaths occur within the first 3–6 h, with more than 50% of the early deaths attributed to uncontrolled hemorrhage [2]. Factors such as uncorrected acidosis, hypothermia, and large crystalloid resuscitation exacerbate coagulopathy, contributing to poor outcomes in trauma patients. Additionally, anticoagulant use further compounds these risk [3,4].
Damage control (DC) anesthesia is a critical component of the comprehensive care provided to patients undergoing DC surgery for acute severe trauma [5], which addresses the hemodynamic challenges of extensive hemorrhage and focuses on appropriate coagulation management in response to trauma-induced coagulopathy (TIC), a unique physiological response to trauma [2]. Recent advancements have demonstrated various pathophysiological mechanisms for TIC and have introduced goal-directed coagulation management as an alternative to traditional ratio therapy [6].
This review aimed to offer comprehensive insights into DC anesthesia, encompassing transfusion and coagulation management, based on an understanding of the pathophysiology of TIC [7]. Additionally, protective strategies for patients with brain injuries, point-of-care viscoelastic monitoring (VEM) in the trauma setting, and effective administrative measures such as massive transfusion protocols or bundle therapy aimed at reducing time and maximizing efficiency have been discussed [6,8].
TRAUMA INDUCED COAGULOPATHY
TIC typically develops within 30 min of sustaining an injury, often preceding a blood transfusion or fluid resuscitation, which has been documented in one-third of all trauma patients and 50–70% of patients with severe trauma [9]. The severity of TIC is directly proportional to the severity of the injury, further increasing the mortality rate of patients by four times than in those without TIC [10]. Early and aggressive coagulation management can effectively improve survival rates and prognosis [2].
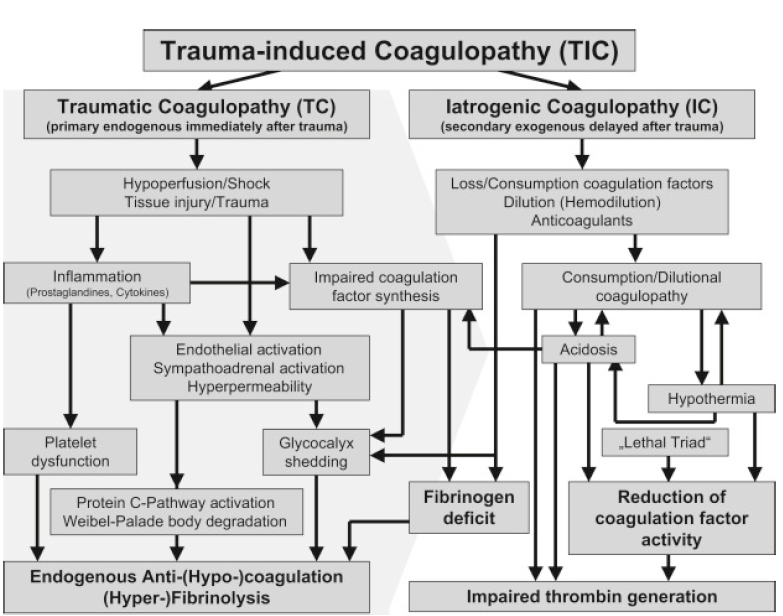
The pathophysiology of TIC is dynamic and multifactorial (Fig. 1). Initially, TIC was attributed to consumption or dilutional coagulopathy caused by massive bleeding or resuscitation. However, the occurrence of TIC within 30 min of injury warranted further explanation for its underlying mechanisms.
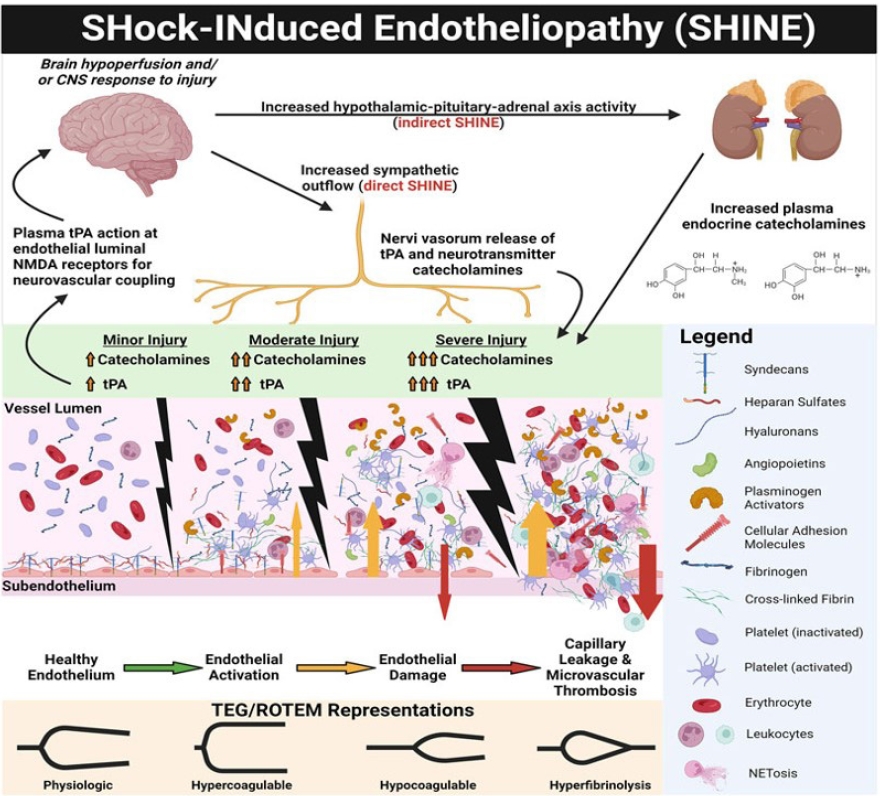
Severe tissue injury accompanied by shock induces systemic vascular endotheliopathy (Fig. 2), which in turn disrupts the body's major defense mechanisms, including cellular immunity, coagulation, and the complement system [11-13]. The glycocalyx is a gel-like layer that covers the luminal surface of vascular endothelial cells and is comprised of membrane-attached proteoglycans, glycosaminoglycan chains, glycoproteins, and plasma proteins [14,15]. Proteoglycans, anchored to the cell membrane, provide structural support for the glycosaminoglycans above them, regulating cellular permeability and vascular tone through the syndecan and glypican families. Heparan sulfate is the most abundant glycosaminoglycan, constituting 50–70% of the total layer. Owing to its large molecular weights (> 70 kDa) and/or negative charge, it maintains the oncotic pressure gradient by hindering the passage of plasma proteins into the cells [10]. Furthermore, natural anticoagulants, such as thrombomodulin and tissue plasminogen activator (t-PA), in the endothelial wall prevent blood cell and protein adhesion, creating an anti-thrombogenic endothelium [16]. Autoheparinization via glycocalyx shedding induces coagulation factors and platelet dysfunction. Activated t-PA and inhibited plasmin-antiplasmin complexes facilitate fibrinolysis. Consequently, fibrinogen depletion and hyperfibrinolysis are prominent features of TIC that are proportional to injury severity [11,13,15,17].
During traumatic shock, nerve endings stimulated by brain hypoperfusion secrete catecholamines and t-PA. Sympathoadrenal stimulation causes glycocalyx damage and the shedding of natural anticoagulants, such as heparan sulfate and thrombomodulin, while activating protein C, t-PA, and platelets [13]. Activated protein C inhibits coagulation factors V and VIII and thrombin production. Activated endothelial walls are susceptible to attachment by platelets, leukocytes, and plasma protein followed by subsequent microvascular thrombosis and augmented leukocyte adhesion [9]. Moreover, autoheparinization by glycocalyx shedding induces dysfunction of coagulation factors and platelets. Various treatments, such as albumin and corticosteroids, and avoiding excessive resuscitation can effectively protect the glycocalyx [12,18].
Several factors, including direct factor loss from excessive bleeding, shock-associated acidosis, hypothermia due to prolonged scene time or excessive fluid resuscitation, age, underlying health status, and prior use of anticoagulant medications, can exacerbate TIC [2,19,20]. In cases of extensive bleeding, a consumption coagulopathy pattern was observed, which was characterized by elevated D-dimer or fibrinogen degradation product (FDP) levels, similar to disseminated intravascular coagulopathy. Tissue factor expression on multiple cell surfaces stimulates systemic coagulation in disseminated intravascular coagulopathy, whereas tissue factors primarily promote thrombus formation at endothelial damage sites in TIC [2]. Trauma patients with early signs of disseminated intravascular coagulopathy, such as increased D-dimer levels, are associated with a poor prognosis [21].
COAGULATION MANAGEMENT
Initial coagulation resuscitation
The rapid reversal of TIC to reduce bleeding is crucial for the prognosis and survival of trauma patients. Appropriate transfusion and coagulation management should be initiated immediately, even before coagulation test results are obtained. It is empirically effective to administer the ratio therapy resembling whole blood for initial coagulation resuscitation [6].
The pragmatic, randomized optimal platelet and plasma ratios trial [22] compared mortality rates and various morbidity outcomes in 680 trauma patients transfused with plasma: platelet: RBC at ratios of 1:1:1 and 1:1:2. Although overall mortality rates did not differ between the two groups, mortality due to exsanguination within 24 h was significantly lower in the 1:1:1 group (9.2% vs. 14.6%; mean difference (MD) –5.4%, 95% confidence interval [CI] –10.4% to –0.5%, P = 0.030). However, there is no clear agreement on the most effective method in a civilized setting. The main strategies for replenishing fibrinogen, a key mechanism in TIC, include the transfusion of fresh frozen plasma (FFP), cryoprecipitate, and hemostatic agents such as fibrinogen concentrate (FC) or prothrombin complex concentrate.
Fibrinogen is a unique precursor of fibrin that cannot be compensated for by other clotting factors. Fibrinogen decrease with decreasing hemoglobin (Hb) levels and is closely related to the amount of bleeding or tissue injury. Insufficient plasma fibrinogen concentrations prevent the formation of hemostatic thrombi with adequate firmness [23]. The binding forces of fibrinogen and fibrin on the platelets are important for the strength of the final clot. In patients with major trauma, the plasma fibrinogen concentration declines faster and more frequently than the other coagulation factors [24]. Concurrent hypofibrinogenemia, even in a prehospital setting, is associated with injury severity and a poor prognosis [2].
Given that the use of prothrombin complex concentrate has not been approved in many countries, including South Korea, FFP is widely preferred for fibrinogen replacement because of its easy availability. However, FFP has a diluting effect owing to its large plasma volume and low efficacy. Therefore, FFP is ineffective in increasing fibrinogen levels above 1.5–2 g/L, which causes a substantial delay in reaching the goal. Additionally, it requires a thawing time of 15–30 min, preventing its concurrent administration at a 1:1 ratio with rapid universal RBC transfusion.
Heo et al. [25] investigated the effect of FC on mortality and blood product requirements in a level 1 trauma center. The administration of FC did not demonstrate surgical benefits but significantly reduced transfusion requirements, including RBCs, FFP, and platelets, in cases of traumatic massive hemorrhages. Furthermore, Innerhofer et al. [26] compared coagulation and clinical outcomes between the FFP (15 ml/kg) and FC (50 mg/kg) groups. Interestingly, compared with the FC group, the FFP group required significantly more rescue therapy, leading to early termination of the study owing to futility and safety concerns (52% vs. 4%, odds ratio [OR] 25.34 [95% CI 5.47–240.03], P < 0.0001). Moreover, the incidence of massive transfusions was significantly higher in the FFP group (30% vs. 12%, OR 3.04 [0.95–10.87], P = 0.042).
Interestingly, the effects of FC supplementation extend beyond fibrinogen replacement and considerably reduce platelet transfusions. In a subsequent study [27] comparing the number of platelet transfusions administered to maintain platelet counts (50–100 × 109 L) between the FFP and FC groups, the FFP group received significantly more platelet transfusions than did the FC group (FFP 47.7% vs. FC 26%, P = 0.0335). Adjusted logistic regression analysis for injury severity scale and traumatic brain injury (TBI) showed that FFP increased the probability of platelet transfusion by 5.79 times (OR 5.79 [1.89–20.62], P = 0.0036), which was even greater than injury severity score (ISS) > 16 (OR 4.33 [2.17–9.74], P = 0.0001). Compared to FFP, FC supplementation exhibited significantly greater platelet-saving effects. In contrast, platelet transfusion failed to increase platelet counts and increased adverse effects [27]. In a study comparing the effects of FC (3 g) and cryoprecipitate (12 units) [18], both methods effectively increased plasma fibrinogen levels. However, FC achieved a greater elevation in fibrin-based clot amplitude (MD of FIBTEM A5:2.6 mm, 95% CI 1.1–4.1 mm, P = 0.001), and the time required to reach the endpoint was reduced by half.
Based on this evidence, the European Guidelines for Massive Hemorrhage and Coagulopathy, sixth edition [6], strongly recommend the combination of FC or cryoprecipitate with PRBCs as an initial coagulation resuscitation. If FC is unavailable, FFP should be administered alongside PRBCs to achieve a fibrinogen level of 1.5–2 g/L as quickly as possible. When using FFP, it is advisable to ensure that the FFP: PRBC ratio is at least 1:2. Since initial coagulation resuscitation usually precedes laboratory or viscoelastic hemostatic assay (VHA) results, clinicians should make rapid decisions based on clinical indicators, such as shock or acidosis.
Coagulation resuscitation should be initiated immediately; however, both FFP and FC require thawing or reconstitution times. To expedite the process for severely injured patients in our trauma center, we administer tranexamic acid (TXA) immediately at a dose of 1 g and activate a massive transfusion protocol automatically requesting blood preparations, including plasma and platelets. Simultaneously, two units of universal donor RBCs are transfused and 3 g of FC is being reconstituted. The patient is promptly transferred to the operation room with reconstituted FC for bleeding control. Therefore, anesthesiologists can ensure rapid administration of coagulation substitutes alongside PRBCs.
Antifibrinolytics
In the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage 2 (CRASH-2) trial [28], the administration of TXA alone significantly reduced overall mortality, highlighting the use of antifibrinolytics as a cornerstone of early treatment in DC resuscitation. Administering TXA within 3 h resulted in a survival benefit. Therefore, this is a simple and cost-effective strategy for early coagulation resuscitation. However, the mortality rates increased when administered after 3 h. In 2019, the CRASH-3 trial was conducted on patients with TBI who were administered TXA within 3 h [29]. Although the method of TXA administration was similar (1 g loading + 1 g maintenance), there was no difference in overall mortality rates. In mild to moderate TBI, a significant reduction in mortality rates was observed (RR 0.78 [95% CI 0.64–0.95], P = 0.005); however, no effect was observed in severely injured patients. The adverse effects related to vascular occlusion were similar despite the use of TXA.
The European Guidelines for Massive Hemorrhage and Coagulopathy, 6th edition in 2023, strongly recommend the intravenous administration of 1 g of TXA at the earliest, ideally within 3 h (even in the prehospital setting), followed by an additional 1 g over 8 h [6]. They emphasize administering it rapidly without waiting for the VHA results.
The administration of TXA to trauma patients has led to questions regarding its appropriate use, particularly regarding its effectiveness in patients with different types of fibrinolysis. Dixon et al. [30] attributed the lack of change in thromboelastography (TEG) results before and after antifibrinolytic administration to the insensitivity of TEG testing, Moore et al. [31] classified patterns of fibrinolysis in trauma patients into the following three types: (1) Physiological type (0.9% < LY 30 < 3.0%), characterized by normal fibrinolysis but increased consumption coagulopathy due to extensive consumption of coagulation factors resulting from trauma; (2) shutdown type (LY 30 < 0.9%), in which plasmin increases alongside the elevation of clotting factors due to trauma, leading to increased fibrinolysis or fibrinogenolysis because of the inhibition of the alpha-2 plasmin activator inhibitor. The shutdown type is more common, occurring in approximately 50% of all trauma patients, and is not detected as hyperfibrinolysis in VHA. Although fibrinolytic activity was previously reported, perfusion was restored and hyperfibrinolysis is not currently observed. Therefore, the shutdown type was not diagnosed as hyperfibrinolysis in the VHA assays; (3) Overt hyperfibrinolysis type (LY 30 > 3.0%) is induced by the shock-mediated release of t-PA from the glycocalyx and can be measured as hyperfibrinolysis in VHA [31,32].
Both types of hyperfibrinolysis can be diagnosed by elevated D-dimer or FDP. The half-life of plasmin or tPA is only a few minutes, but that of D-dimer, FDP, and the plasmin-antiplasmin complex is 12–16 h. Therefore, despite the lack of ongoing hyperfibrinolysis, patients with the shutdown type exhibit elevated levels of d-dimer or FDP in their blood, without hyperfibrinolytic evidence on TEG.
The use of antifibrinolytics in shutdown type remains controversial. The shutdown type does not benefit from antifibrinolytics and has a poor prognosis in terms of mortality and bleeding compared with those with normal fibrinolysis [33]. Mortality rates were higher in the overt hyperfibrinolysis type (44%) and shutdown type (17%) than in the physiologic group (3%) (P = 0.001). The main causes of death in the shutdown type were predominantly TBI (45%) and hemorrhage (42%), with only 13% attributed to multiple organ failure. Interestingly, hemorrhage remained a major cause of death, even in the shutdown type. Overt hyperfibrinolysis type was associated with hypoperfusion (shock) in both non-TBI and TBI patients, resulting in higher mortality rates and worse outcomes. However, hyperfibrinolysis can occur without hypoperfusion in TBI patients [23].
The widely recommended use of antifibrinolytics, known for their low cost and minimal adverse effects, may need to be carefully re-evaluated for the risk of thromboembolic events or multiple organ failure, especially in patients with shutdown or physiologic types. Further comprehensive studies are warranted to effectively guide clinical practice.
Platelets
Platelets are vital for hemostasis; however, the effects of platelet count and platelet transfusion on coagulation and clinical outcomes in severe trauma have not been elucidated [22]. However, the preemptive administration of platelets as part of the fixed-ratio blood product strategy for massive hemorrhage remains controversial. Although some studies have suggested that platelet transfusion reduces mortality and bleeding [34], others have indicated that it does not increase platelet count or significantly affect bleeding volume [27]. Additionally, the findings suggest that prophylactic platelet transfusion in patients on antiplatelet therapy may elevate thromboembolic risk, adding complexity to the ongoing debate on platelet transfusion in ratio therapy.
Moreover, with emerging research suggesting that FC alone may sufficiently enhance clot strength, interest in both prophylactic and therapeutic platelet transfusions has waned. Lang et al. [24] measured the maximal clot strength according to the amount of fibrinogen using FIBTEM under various settings while controlling the platelet count. The clot strength decreased when the platelet count was less than 100 × 103 mm3 and reached a plateau at 400 × 103 mm3. Additionally, even when the platelet count is below 100 × 103 mm3, increasing the dose of fibrinogen results in an increase in clot strength. Conversely, regardless of the platelet count, clot strength increased in proportion to the fibrinogen concentration. Because one platelet contains many fibrinogen receptors (40,000–50,000 copies per platelet), a large amount of fibrinogen can successfully increase clot strength even when the platelet count is < 20 × 103 mm3. In moderate thrombocytopenia, near-normal maximal clot firmness values (reference range, 54–72 mm) were achieved with fibrinogen at 400–600 mg/dl, whereas in severe thrombocytopenia, near-normal maximal clot firmness values were reached only with fibrinogen above 600 mg/dl [24].
Furthermore, when comparing the amount of platelet transfusion required to maintain platelet counts between 50–100 × 109 L as part of the secondary coagulation strategy between the FFP group and FC group, the FFP group had a significantly higher requirement (47.7% vs. 26%, P = 0.0335) [27]. Adjusted logistic regression analysis for stratification variables such as ISS and brain injury revealed that FFP therapy increased the probability of platelet transfusion by 5.79 times (OR 5.79 [1.89–20.62], P = 0.0036), which was even higher than ISS > 16 (OR 4.33 [2.17–9.74], P = 0.0001). Overall, although FC supplementation exhibited significantly greater platelet-saving effects than FFP, platelet transfusion itself did not effectively enhance platelet counts and resulted in poor outcomes [27]. Nevertheless, the sixth edition of the European Guideline for Massive Hemorrhage and Coagulopathy recommends that if platelet transfusion is deemed necessary, it should be performed initially with a high platelet: PRBCs ratio [6].
Further goal directed coagulation resuscitation
The effectiveness of goal-directed coagulation management guided by point-of-care VEM or conventional coagulation assays has been reported [24, 35]. In particular, compared to conventional coagulation assays, which only reflect procoagulant activity, VEM is a specialized and specific point-of-care test that rapidly identifies key components of TIC, such as hypofibrinogenemia and hyperfibrinolysis [14]. A protocolized algorithm for VEM-guided coagulation management was successfully developed and implemented [35]. The introduction of VEM-based coagulation management has led to a change in the pattern of blood product utilization and has had a substantial impact on patient outcomes. These include quicker decision-making and initiation of coagulation therapies, improvements in coagulation parameters, safer transfusion strategies, and blood conservation [14]. Moreover, compared to conventional coagulation assays, the survival rates were higher while also reducing overall costs by necessitating less plasma and platelets. In a level 1 trauma center study [14], there was a significant increase in the number of patients receiving cryoprecipitate (P = 0.010) with the introduction of rotational thromboelastometry (ROTEM). Moreover, the subsequent use of platelets and FFP significantly decreased in patients receiving cryoprecipitate (P < 0.001).
Conventional coagulation assays can be used when VEM is unavailable. In instances where the INR or aPTT increases to 1.5 times the normal range, goal-directed coagulation management can be initiated [6]; FC is preferred over FFP. The goal was to achieve a plasma fibrinogen concentration of 1.5–2 g/L by administering 3–4 g of FC (or 15–20 units of cryoprecipitate) and reassessing it through VEM or Conventional coagulation assays [6].
Although the platelet count is initially maintained at near normal levels in the early stages of trauma, platelet aggregating function is often impaired. Several guidelines recommend early and high-ratio platelet transfusion, and the platelet transfusion trigger is set at a platelet count above 50 × 109 L for trauma patients experiencing ongoing bleeding and above 100 × 109 L for patients with TBI [6]. However, in the early phase of trauma, platelet aggregation usually does not recover even with platelet transfusion [4]. This injury-related initial resistance period to platelet transfusion gradually recovers over time, with an increase in platelet aggregation observed at 48–96 h [36]. Consequently, the effectiveness of platelet transfusion increases over time. Moreover, platelet transfusion in TBI patients on antiplatelet therapy often impairs platelet function and increases thromboembolic complications [4]. Therefore, the need for early and high-ratio platelet transfusion is controversial, and prophylactic platelet transfusion based on POC platelet mapping or platelet transfusion in patients taking antiplatelet agents is not recommended [6]. If administered, the guidelines recommend an initial dose of 4–8 single platelet units or one aphaeresis pack [6].
Calcium
Ionized calcium (Ca2+) plays a pivotal role in the coagulation cascade [37]. Ca2+ is essential for the formation and stabilization of fibrin polymerization sites and for various platelet-related functions. Moreover, Ca2+ acts as a cofactor in the activation of factors II, VII, IX, and X, as well as proteins C and S. Reduced Ca2+ levels can impair cardiac contractility and systemic vascular resistance. During massive transfusions in trauma, hypocalcemia occurs due to the chelation of serum Ca2+ by citrate, which is used as a preservative and anticoagulant in blood bags. Calcium chloride is the preferred option for correcting hypocalcemia, as it provides 270 mg of elemental calcium is present in a 10 ml solution of 10% calcium chloride. In contrast, 10 ml of 10% calcium gluconate provides only 90 mg of elemental calcium [6].
DAMAGE CONTROL ANESTHESIA
Based on the DC concept, anesthesiologists play a crucial role in trauma care by collaborating closely with the surgical team to ensure optimal surgical conditions during DC surgery and correct any physiological imbalances under DC resuscitation with the aim of maintaining adequate perfusion to vital organs. This involves not only performing procedural tasks, such as airway management and vascular access, but also providing comprehensive anesthesia care, including vigilant monitoring of coagulation status and perfusion as well as massive transfusions and coagulation management [5].
DC concept
Performing definitive surgery directly on patients with severe hemorrhagic trauma remains a major challenge. DC is a treatment strategy that is beneficial for patients with severe trauma [7]. The DC concept is based on a sequential treatment strategy that prioritizes physiological restoration over definitive surgery. This concept comprises three stages: first, emergency surgery to control bleeding and contamination; second, adequate physiological stabilization to address acidosis, hypothermia, and coagulopathy in the intensive care unit (ICU) (referred to as DCR); and third, definitive repair surgery performed after 24–48 h [7].
The concept of DC must be continued until the patient is saved, and the patient's physiology must be managed based on the principles of DCR, even during anesthesia. DCR is based on specific resuscitation goals related to early and aggressive hemostasis, treatment, and prevention of the lethal triad of acidosis, hypothermia, and coagulopathy (Table 1).
Hemoglobin levels
The restrictive transfusion threshold uses a lower Hb concentration as the transfusion threshold (typically 7.0–8.0 g/dl), whereas the liberal transfusion threshold employs a higher Hb concentration as the transfusion threshold (typically 9.0–10.0 g/dl). Restrictive transfusion reduced the proportion of individuals requiring RBC transfusion by 41% across a wide range of clinical scenarios, with no difference in mortality or morbidity [38]. Additionally, several studies have demonstrated better neurological outcomes with a Hb threshold of 7.0 g/dl than with 10.0 g/dl [39]. Therefore, RBC transfusion should target a Hb level of 7.0–9.0 g/dl [6].
Blood pressure management
Restricted volume replacement and permissive hypotension are preferred for the initial management of trauma-induced hypotension [6]. If there are no clinical signs of brain injury, restricted volume replacement strategy with a target systolic blood pressure of 80–90 mmHg (mean arterial pressure: 50–60 mmHg) is recommended until major bleeding stops [40]. Before the recent adoption of early and high plasma and platelet ratio therapies, resuscitation for acute trauma typically involved large volumes of crystalloids and RBC transfusion. The traditional aggressive volume replacement of crystalloids aimed at normal blood pressure not only increases mortality but also leads to complications such as adult respiratory distress syndrome, abdominal compartment syndrome, coagulopathy, multiorgan failure, hospital-acquired infections, increased need for transfusions, and prolonged ICU and hospital stays [3]. However, permissive hypotension and restricted volume resuscitation should be avoided in patients with TBI or spinal cord injury to ensure adequate perfusion of the central nervous system.
Hypothermia treatment
Hypothermia in trauma patients is a condition referring to a core temperature < 36°C, which increases mortality and morbidity and the amount of bleeding [20, 38]. A study comparing patients with normal body temperature (36–38.5°C) with those with hypothermia (< 36°C) at a level 1 trauma center reported that two-thirds of patients exhibited hypothermia [20]. An estimated 1° decrease in temperature resulted in a 10% increase in RBC transfusion within the first 24 h. The presence of hypothermia upon arrival was identified as an independent predictor for 24 h mortality (OR 2.7, 95% CI 1.7 – 4.5, P < 0.001), which significantly elevated 30-day mortality (OR 1.8, 1.3–2.4; P < 0.001) [20].
Improving neurological outcomes can be particularly challenging in patients undergoing therapeutic hypothermia. However, induced hypothermia aggravates the status of TIC by reducing platelet function and coagulation factors and inducing hyperfibrinolysis. Moreover, hypothermia increases the development of hypotension, acidosis, and postoperative pneumonia [40]. Target temperature management has been implemented for brain protection in patients after shock or cardiac arrest [41], which involves maintaining a steady temperature between 33–36°C for over 24 h, followed by a slow temperature increase of less than 0.5°C per hour. The goal was to prevent fever (temperature > 38°C). Because target temperature within the range of 33–36°C showed no difference in neurological prognosis, maintaining a temperature of 36°C is preferred, especially in patients with severe hemorrhagic trauma. Patients transferred to the operating room for surgery are often in a severely hypothermic state and should be actively warmed to reach the target temperature.
Bleeding control bundle-of-care
A series of measures to optimize bleeding control, including accurate identification of the bleeding patient; DC resuscitation; hemostatic techniques with tourniquets, pelvic binders, or hemostatic dressings; resuscitative endovascular balloon occlusion of the aorta; VEM; TXA administration for substantial hyperfibrinolysis; decreased time to the operating room and interventional radiology; and goal-directed resuscitation with blood products [8].
Mortality rates in a level 1 trauma center were compared between 2005–2006 and 2012–2013, which reported that TBI and bleeding account for > 91% of all deaths. Although no difference was observed in other causes, deaths due to bleeding decreased from 36 to 25%, which was attributed to the impact of the bleeding control bundle therapy. Of all deaths, 60% occurred within 24 h and 50% occurred within 1 h. The leading causes of death were TBI (61%), bleeding (36–25%, P < 0.01), and multiple organ failure or sepsis (6.2%).
Neuroprotection in patients with TBI
To ensure neuroprotection in TBI patients, it is essential to maintain adequate oxygen delivery to ischemic areas while avoiding hypoxia. However, excessive hyperoxia (> 450 mmHg) can also pose risks, such as oxygen toxicity, vasoconstriction, and free radical injury [42]. Although hyperoxia is uncommon in trauma conditions such as pulmonary trauma, systemic inflammatory changes, or excessive fluid resuscitation, continuous monitoring is essential to ensure adequate oxygenation and prevent hypoxia. With pulse oximetry and blood gas analysis, noninvasive monitoring of the oxygen reserve index can be helpful in this regard [43].
Permissive hypotension and restricted volume resuscitation are contraindicated to ensure adequate perfusion of the injured central nervous system in patients with TBI and spinal cord injuries. For patients with severe TBI (GCS ≤ 8), maintaining a mean arterial pressure ≥ 80 mmHg is recommended. In older patients with trauma and chronic hypertension, blood pressure management to prevent hypoperfusion is essential [44]. If the target blood pressure is not achieved with a restricted volume replacement strategy, administering noradrenaline in addition to fluids is advisable to maintain the target mean arterial pressure [6].
Ventilation should maintain normocapnia with a PaCO2 of 35–40 mmHg unless it is an emergency where brain herniation is imminent. Particularly within the first 24 h, stabilizing cerebral blood flow by maintaining a consistent PaCO2 level is important.
In addition, hypothermia is associated with increased mortality, hypotension, acidosis, and worsening of TIC (10% decrease in function per 1°C decrease in temperature); therefore, hypothermic treatments, whether therapeutic or prophylactic, should be avoided [40]. Instead, active warming is pursued with a target temperature management of 36°C.
Several studies have demonstrated an association between anemia and mortality or poor outcomes [38,40,45], but most of these studies were retrospective, and the Hb threshold has not been established. In the normal brain, a gradual decrease in Hb can be compensated for by cerebral vasodilation, leading to an increased cerebral blood flow (CBF), and compensation can occur up to a threshold Hb level of approximately 5–6 g/dl. At this point, maximum CBF is achieved, and cerebral oxygen delivery gradually decreases as further vasodilation is limited. However, lower CBF was observed for similar Hb levels in the injured brain than in the normal brain. Owing to impaired cerebrovascular reserve, maximum vasodilation may occur at an Hb level of approximately 8–9 g/dl, and a further decrease in Hb below this threshold may contribute to a decrease in cerebral oxygen delivery. However, both anemia and high Hb levels can increase viscosity and worsen CBF. Therefore, maintaining Hb levels at 7–9 g/dl is recommended [38].
Thromboprophylaxis
Upon achieving hemostasis, the critical care focus should shift towards hypercoagulability, preparing for events such as thromboembolic events or multiple organ failure. The risk of thromboembolic events after traumatic hemostasis is considerably high, with pulmonary embolism being the third leading cause of death after the third day [46]. For patients who are immobile and at risk of bleeding, mechanical thromboprophylaxis with intermittent pneumatic compression should be initiated at the earliest. If bleeding is controlled within 24 h, combined pharmacological and intermittent pneumatic compression thromboprophylaxis should be implemented until the patient is mobile [46]. However, the routine use of graduated compression stockings or inferior vena cava filters for thromboprophylaxis is not recommended because of their lack of efficacy.
CONCLUSION
Severe tissue injury and hemorrhagic shock result in TIC, necessitating early and aggressive coagulation resuscitation. Initial management should prioritize antifibrinolytics and fibrinogen supplementation guided by clinical indicators such as shock or acidosis, even before obtaining laboratory results. Subsequent goal-directed coagulation management should be maintained using viscoelastic assays or coagulation tests. FC shows promise for its rapid and robust efficacy, lack of transfusion reactions, and ability to enhance clot strength, particularly in cases of severe platelet dysfunction. Ongoing research in severe trauma and standardized damage control anesthesia may lead to transfusion-free, goal-directed coagulation management and reduce preventable bleeding deaths.
This comprehensive review provides valuable insights for a seamless transition from the trauma bay to the intensive care unit, addressing disrupted physiology and ensuring efficient hemodynamic and coagulation resuscitation.
Notes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Min A Kwon. Writing - review & editing: Min A Kwon, Sung Mi Ji.