Pediatric lung ultrasound: its role in the perioperative period
Article information
Abstract
There have been a number of recent advancements in the field of point-of-care ultrasound, including lung ultrasound for adult and pediatric populations. Evidence-based guidelines on the use of point-of-care lung ultrasound have been published. Lung ultrasound is superior to chest radiography in diagnosing several disorders of vital importance in the perioperative period. This review presents a discussion of techniques and clinical applications of lung ultrasound in pediatric patients focusing on usage in the perioperative period.
INTRODUCTION
Traditionally, the lungs were considered inappropriate for ultrasound examination because of the bony cage and the presence of air within the lungs. However, in the late 1900s, Lichtenstein et al. [1–4] initiated a new era of lung ultrasound, and reported that artifacts and signs produced by ultrasound beams that penetrate the lungs may have some clinical significance. Since Copetti et al. [5] first using lung ultrasound to diagnose transient tachypnea of newborns in 2007, several reports highlighting the application of lung ultrasound for diagnosing pulmonary diseases such as respiratory distress syndrome, meconium aspiration syndrome, pneumonia, bronchiolitis, and atelectasis in pediatric patients have been published [6–12]. Point-of-care ultrasound is rapidly evolving and becoming the standard of care in numerous clinical situations, such as vascular access in the operating room [13]. The 2012 international evidence-based recommendations for point-of-care lung ultrasound introduced its role in the pediatric population [14]. This review presents current knowledge regarding lung ultrasound in the pediatric population focusing on the perioperative period.
BASIC PRINCIPLES OF LUNG ULTRASOUND
Before performing lung ultrasound, it is important to note that the position of the patient could affect the findings, as air increases and liquid sinks under the influence of gravity. During the perioperative period, lung ultrasound is usually performed with the patient in the supine position. While lung consolidation or pleural effusion is predominantly found in dependent and dorsal lung regions, pneumothorax is primarily found in the anterior chest. However, there is variability in the scanning technique, and as yet there is no standardized protocol for the pediatric population. In children, each hemithorax can be divided into several regions, and different scanning schemes have been suggested [15,16]. A recent editorial recommended that at least six quadrants of each hemithorax should be scanned for a complete lung ultrasound examination [17]. Fig. 1 shows an example of lung ultrasound scanning technique in a pediatric patient. In a given region of interest, the lung surface of all of the adjacent intercostal spaces must be thoroughly explored by moving the probe transversally [18]. Although most transducers can be used for lung ultrasound, the most suitable probe depends on the size of the patient and the suspected pathology. In the pediatric population, a high-frequency (5–15 MHz) linear probe or microconvex probe is suitable for evaluating both superficial and deep pathologies. Although most scanning is performed in two-dimensional mode, motion mode (M-mode) and color Doppler can also be used depending on the suspected pathology [19].
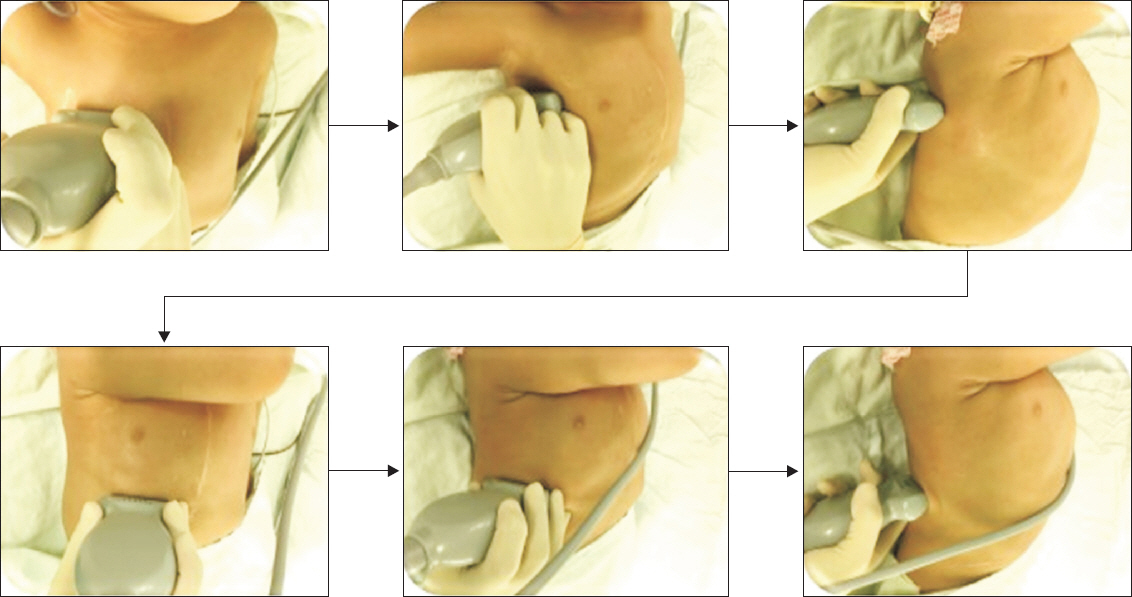
Lung ultrasound scan in a 2-year-old boy using a high-frequency (5–10 MHz) linear transducer. Each hemithorax is divided into six regions using three longitudinal lines (parasternal, anterior, and posterior axillary) and two axial lines (one above the diaphragm and the other 1 cm above the nipples). Twelve regions of the lungs are scanned sequentially from right to left, cranial to caudal, and anterior to posterior. The figure shows scanning of the right hemithorax.
LUNG ULTRASOUND FINDINGS
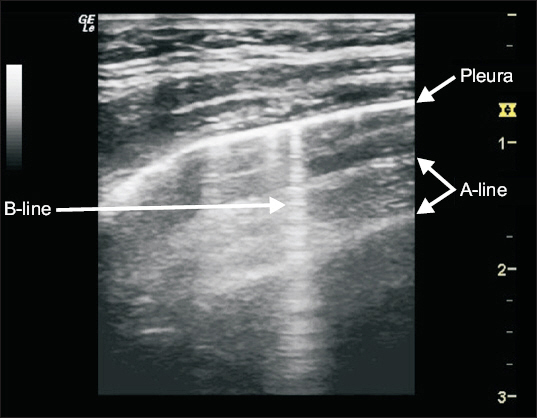
Lung ultrasound begins by understanding artifacts and signs that arise from the pleura. Several studies have previously illustrated normal lung ultrasound findings in the pediatric population [15,20]. Notably, ultrasound beams are not transmitted via a normally aerated lung. Only the pleura, which appears as a hyperechoic and sliding line moving forward and backward with ventilation, can be observed. Beyond this pleural line, motionless and regularly spaced horizontal lines to the pleura, known as A-lines, are visualized, corresponding to standard reverberation artifacts of the pleural line. Both lung sliding and A-lines define normal aeration [18]. By contrast, vertically oriented artifacts, called B-lines, indicate an abnormality in the interstitial or alveolar compartment and are correlated with the lung interstitial fluid content. Moreover, B-lines project from the pleural line to the edge of the screen, erase A-lines, and move with respiration (Fig. 2) [15].
PERIOPERATIVE LUNG ULTRASOUND IN PEDIATRIC PATIENTS
Lung ultrasound has shown good performance in diagnosing lung consolidation, pleural effusion, pneumothorax, and alveolar interstitial syndrome [20,21], which are common during the perioperative period as underlying conditions or complications. Therefore, anesthesiologists should be familiar with lung ultrasound findings of these pathologies.
Lung consolidation
Lung ultrasound is useful for differentiating among atelectasis, pneumonia, and mass lesions. A typical feature of lung consolidation is a liver-like, hypoechoic echotexture with strip-like air or/and fluid bronchograms [22]. In addition, lung consolidation usually shows an irregular border (shred sign), and multiple B-lines or white lung can be observed because of abundant B-lines (Fig. 3). The incidence of anesthesia-induced atelectasis is significantly higher in pediatric patients than in adult patients. Acosta et al. [16] reported that the most prevalent lung ultrasound findings of anesthesia-induced atelectasis in children are juxtapleural consolidations, followed by the absence of A-lines, presence of B-lines, and air bronchograms.
Pleural effusion
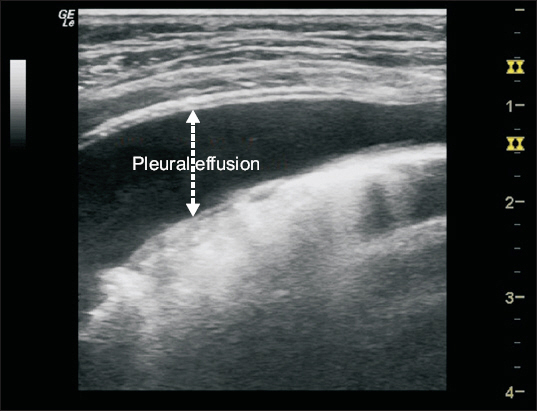
The use of ultrasound for diagnosing pleural effusion, as well as for guidance of thoracentesis, is the most established application of lung ultrasound. On lung ultrasound, pleural effusion appears as an anechoic space between the parietal and visceral pleurae (Fig. 4). The lung behind the effusion displays different degrees of aeration [17]. Although simple effusion is homogeneous, complex effusions, such as a hemothorax or empyema, are heterogeneous [19].
Pneumothorax
In the case of pneumothorax, air is present between the parietal and visceral pleurae. As air completely reflects ultrasound waves, the visceral pleura is not observed, and there is no lung sliding. At the border of the pneumothorax, the pleural interface should be intact, and normal lung sliding should be present. This transition point between the intermittent presence and absence of lung sliding is called the “lung point” (Fig. 5), which is a pathognomonic sign of the presence of a pneumothorax. The use of M-mode facilitates the exclusion of pneumothorax. If pneumothorax is present, the absence of lung sliding results in a series of black and white horizontal lines, which are called the “stratosphere or barcode” sign [19].
Alveolar interstitial syndrome
B-lines are the principal characteristic of alveolar interstitial disease. In addition, increased B-lines on lung ultrasound are attributed to the increased extravascular lung water. As only a few B-lines are visible in healthy subjects, the presence of multiple B-lines suggests an interstitial lung syndrome. However, this is nonspecific regarding etiology and may be found in various conditions such as pulmonary edema, acute respiratory distress syndrome, or pulmonary contusion [22].
Assessment of perioperative hypoxia
Perioperative hypoxia can directly affect prognosis, with pediatric patients being more vulnerable to such hypoxia [23]. Therefore, it is imperative to immediately assess the cause of perioperative hypoxia in pediatric patients. The sensitivity and specificity of lung ultrasound are similar to those of computed tomography and surpass those of chest X-ray for the pathological conditions mentioned above [20,21]. Piette et al. [19] proposed a protocol for investigating patients with hypoxia, whereas Lichtenstein [24] proposed a method for exploring acute respiratory failure. Nevertheless, anesthesiologists should be cautious when applying these protocols in pediatric patients because they were developed based on data obtained from adults.
LIMITATIONS AND FUTURE PERSPECTIVES
Despite the proven diagnostic power of lung ultrasound and its impact on decision-making and therapeutic management, there are still significant barriers to the widespread adoption of this tool [17]. Primary challenges in implementing lung ultrasound in daily anesthetic practice are its operator dependency and variable expertise in skill among anesthesiologists. Although lung ultrasound has great promise with the establishment of correct training and accreditation processes, it remains in its infancy, particularly in the pediatric population [21]. The development of a validated lung ultrasound program is mandatory and requires a combination of dedicated personnel, adequate instrumentation, and training protocols [17].
CONCLUSIONS
Lung ultrasound is gaining acceptance among anesthesia providers because its application has improved the diagnostic accuracy of potentially life-threatening conditions during the perioperative period. The noninvasive nature of this technique, lack of radiation exposure, and easy access make it a feasible imaging modality for perioperative care [25]. Due to the sensitivity of pediatric patients to radiation hazards and patient transfer, lung ultrasound could be a favorable option or use in the pediatric population. Overall, lung ultrasound is a contemporary skill that anesthesiologists should strongly consider incorporating into their clinical practice.