INTRODUCTION
The number of elderly people in Korea has been quickly increasing from 10.2% in 2010 to 12.3% in 2015 [
1]. They accounted for 39% of the total number of surgeries performed in 2015 [
2]. Older patients have a higher occurrence of disease-associated and drug-associated changes in physiology [
3]. Perioperative management including intraoperative sedation in older patients need to be carefully considered because aging-related physiological changes are individualized and progressive.
Adjustment of drug dosages may be necessary because the pharmacokinetic and pharmacodynamic changes in drugs and the large variability in the physiological changes in elderly individuals may increase the risk for adverse drug reactions in some patients [
4,
5]. Elderly patients may be at risk of overdose if doses are based on actual body weight (ABW). Therefore, doses must be carefully regulated to suit each elderly patient. Dose adjustment based on dosing scalars such as ideal body weight (IBW), ABW, and lean body mass may be used for specific drugs. However, many clinicians have probably used subjective, unscientific methods of dose reduction to avoid overdose in elderly patients.
Dexmedetomidine (DEX) has sedative and analgesic properties associated with a reduction in the use of anesthetics without significant respiratory depression when the dosage administered is within the therapeutic range [
6]. However, DEX has a longer onset compared to other sedative agents [
7] and rapid administration or a high dose might produce side effects including bradycardia, hypotension, and hypertension [
8].
The aim of this study was to compare several dosing schedules of intravenous DEX and identify the appropriate dosing schedules for adequate sedation of elderly patients under spinal anesthesia in the therapeutic range.
MATERIALS AND METHODS
Our Institutional Ethics Committee approved this study. Written informed consent was obtained from all patients. A total of 120 elderly patients who were Ōēź 65 years of age, had a body mass index (BMI) Ōēż 30 kg/m2, were classified as American Society of Anesthesiologists (ASA) physical status (PS) I and II, and were scheduled for lower extremity operation under spinal anesthesia were recruited. Patients who had refused to be included in the study and those with a bleeding tendency, cardiac arrhythmia, a psychiatric disorder, a history of sleep apnea and airway obstruction, recent administration of sedative drugs or ╬▒-adrenergic antagonists, age < 65 years, or BMI > 30 kg/m2 were excluded.
The patients were arbitrarily divided into three groups (40 patients each) using computer-generated random numbers. Patients in Group A (n = 40) were administered a loading dose of 1.0 ╬╝g/kg of ABW and a maintenance dose of 0.5 ╬╝g/kg of ABW/h. Patients in Group B (n = 40) were administered a loading dose of 1.0 ╬╝g/kg of IBW and a maintenance dose of 0.5 ╬╝g/kg of IBW/h. Patients in Group C were administered a loading dose of 0.8 ╬╝g/kg of IBW and a maintenance dose of 0.5 ╬╝g/kg of IBW/h (
Fig. 1). Patients were blinded to the group allocation, but the researcher and observer were not blinded. IBW in kg was calculated using the Devine formula (male: 50 + 0.91 [height in cm ŌĆö 152.4 cm]; female: 45.5 + 0.91 [height in cm ŌĆö 152.4 cm]).
Fig.┬Ā1

None of the patients received premedication. After the patient arrived in the operating room, we began routine intraoperative monitoring including electrocardiogram, noninvasive blood pressure measurement, pulse oximetry (SpO2), and bispectral index score evaluation (BIS; A-2000, Aspect Medical Systems, USA). About 400-500 ml of crystalloid solution was administered to a patient before injection of spinal anesthetics. With the patient in the right or left lateral recumbent position, spinal puncture was performed through the L3-4 or L4-5 level using the midline or paramedian approach technique. A dosage of 0.5% heavy bupivacaine was determined based on the anesthesiologistŌĆÖs personal experience and on the patientŌĆÖs characteristics. After the anesthetic drug was injected, the patientŌĆÖs position was promptly changed to the supine position. The targeted sensory level (T8-T10) of anesthesia was checked using four consecutive pin tests. After the targeted sensory anesthesia was identified, an initial loading dose of DEX was infused over 10 minutes, and then changed to the maintenance infusion dose. During the study, the target adequate sedation level was set at BIS 60-80. All patients spontaneously breathed and 100% oxygen was supplied via nasal prong at a rate of 3 L/min throughout the operation.
The patientŌĆÖs vital signs and SpO2 were continuously monitored and recorded every 5 min. Before DEX infusion, we recorded BIS and airway obstruction score (1: patent airway; 2: airway obstruction relieved by neck extension; 3: airway obstruction requiring jaw retraction). We rechecked these items after the loading dose infusion (10 min). After that, we checked them every 20 min. The time required to reach BIS 80 was recorded separately. If the patient became bradycardic (heart rate < 50 beats/min), atropine sulfate 0.5 mg was injected intravenously for treatment. This study was conducted for a total of 70 min. After the study was completed, infusion doses were re-adjusted for adequate sedation.
Statistical analysis
We included a total of 120 patients with 40 patients in each group. Data are shown as mean (or median) ┬▒ standard deviation or the actual value. Statistical analysis was performed using SPSS software (SPSS version 21.0, IBM Corp., USA). Normality of age, height, ABW, initial loading dose, and the time required to reach BIS 80 was evaluated using the Kolmogorov-Smirnov test; we used a nonparametric test when the assumption of normality was violated. In addition, the normality of BIS over time was evaluated using the Kolmogorov-Smirnov test. We used the Mauchly test for sphericity and Greenhouse-Geisser epsilon for correction of the significance if the assumption of sphericity was violated.
Sex and the frequency of bradycardia were statistically analyzed using the Chi-square test. ASA PS was evaluated using FisherŌĆÖs exact test. The comparisons of IBW and ABW within each group were statistically analyzed using a paired t test. Age, height, ABW, initial loading dose, and the times required to reach BIS 80 among the groups were analyzed using a one-way ANOVA test (TukeyŌĆÖs honestly significant difference [HSD] post-hoc test). Two-way ANOVA with repeated measures in one factor test (TukeyŌĆÖs HSD post-hoc test) was used to evaluate BIS among the groups. Airway obstruction scores among three groups at the same time were evaluated using the Chi-square test and airway obstruction scores between two groups at the same time were evaluated using the Mann-Whitney U test. P < 0.05 was considered statistically significant.
RESULTS
All 120 patients, with 40 patients per group, were recruited and all the included patients completed the study.
Age, height, ABW, and initial loading dose showed normality. Sex, age, ASA PS, height, IBW, and ABW among the groups were not statistically different. The IBW of patients were less than the corresponding ABW in all groups (P < 0.001 in all groups) (
Table 1). The difference in loading dose of DEX among the groups was statistically significant (P < 0.001 [group A vs. B, group A vs. C, and group B vs. C: P < 0.001, in all groups]).
Table┬Ā1
|
ŌĆāGroup A (n = 40)ŌĆā |
ŌĆāGroup B (n = 40)ŌĆā |
ŌĆāGroup C (n = 40)ŌĆā |
|
Sex (M/F) |
7/33 |
7/33 |
6/34 |
|
Age (yr) |
72.0 ┬▒ 5.4 |
72.6 ┬▒ 5.7 |
74.1 ┬▒ 6.6 |
|
ASA (I/II) |
4/36 |
6/34 |
2/38 |
|
Height (cm) |
154.2 ┬▒ 8.1 |
150.3 ┬▒ 8.5 |
152.0 ┬▒ 5.8 |
|
Body weight (kg) |
|
|
|
|
ŌĆāIBW |
48.0 ┬▒ 8.3 |
47.7 ┬▒ 9.4 |
46.0 ┬▒ 6.2 |
|
ŌĆāABW |
62.4 ┬▒ 8.5*
|
59.1 ┬▒ 11.6*
|
60.8 ┬▒ 9.1*
|
|
Loading doses (mg)ŌĆĀŌĆā |
62.4 ┬▒ 8.5 |
47.7 ┬▒ 9.4 |
36.8 ┬▒ 4.9 |
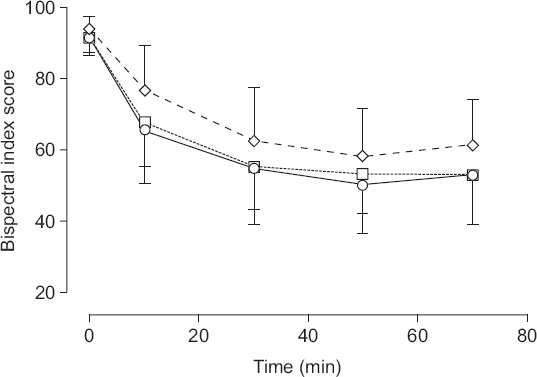
The BIS over time showed normality but violated the Mauchly test of sphericity (Group A: P = 0.021; Group B: P = 0.033; Group C: P < 0.001). However, BIS over time showed significant DEX effect and showed linear trend by Greenhouse-Geisser epsilon (P < 0.001). BIS among the groups over time was statistically different (P < 0.001 [group A vs. B, P = 0.490; group A vs. C, P = 0.001; group B vs. C, P = 0.026]). Group A and B had lower BIS than the adequate sedation level from 30 min after initial loading dose to the end of this study (
Fig. 2).
Fig.┬Ā2
Bispectral index score. Circle represents group A (loading dose of dexmedetomidine: 1 ╬╝g/kg of actual body weight). Square represents group B (loading dose of dexmedetomidine: 1 ╬╝g/kg of ideal body weight). Triangle represents group C (loading dose of dexmedetomidine: 0.8 ╬╝g/kg of ideal body weight). After administration of the loading dose in all groups, a maintenance dose of 0.5 ╬╝g/kg of ideal or actual body weight/h was administered. Data represents mean ŌĆö SD in groups A and B, and mean + SD in group C. Group A vs. B, P = 0.490; group A vs. C, P = 0.001; group B vs. C, P = 0.026.
Because the time required to reach BIS 80 did not show normal distribution, we used the Kruskal-Wallis test for statistical analysis among groups and the Mann-Whitney U test for statistical analysis between groups. The times required to reach BIS 80 are expressed as median ┬▒ standard deviation and were 6.1 ┬▒ 5.3 min, 5.0 ┬▒ 3.6 min, and 11.0 ┬▒ 8.6 min in groups A, B, and C, respectively (P < 0.001 [group A vs. B, P = 0.31; group B vs. C, P < 0.001; group A vs. C, P = 0.002]).
The airway obstruction score among the groups was not statistically different except during the loading dose infusion (P = 0.016) (
Table 2). The frequency of bradycardia (2 cases in group A, 3 cases in group B, and 3 cases in group C) was not statistically different among the groups.
Table┬Ā2
|
Group A (n = 40) |
Group B (n = 40) |
Group C (n = 40) |
P value |
|
0-10 min after infusion |
31/9/0 |
31/8/1 |
40/0/0 |
0.016 |
|
11-30 min after infusion |
32/4/4 |
30/10/0 |
32/7/1 |
0.097 |
|
31-50 min after infusion |
31/5/4 |
30/9/1 |
32/7/1 |
0.379 |
|
51-70 min after infusion |
35/3/2 |
30/8/1 |
32/7/1 |
0.515 |
DISCUSSION
After the BIS reached 80, the mean BIS in group C, with a loading dose of 0.8 ╬╝g/kg of IBW and a maintenance dose of 0.5 ╬╝g/kg of IBW/h, was maintained at adequate sedation levels during the study except at 50 min after the start of infusion. However, group A used a loading dose of 1.0 ╬╝g/kg of ABW and a maintenance dose of 0.5 ╬╝g/kg of ABW/h, and group B used a loading dose of 1.0 ╬╝g/kg of IBW and a maintenance dose of 0.5 ╬╝g/kg of IBW/h, which resulted in BIS that was lower than the adequate sedation level from 30 min after initial loading dose to the end of this study. Patients in group C took more time to reach BIS 80 than those in groups A and B. The airway obstruction score and the frequency of bradycardia among the groups were not statistically different.
Although DEX has been used for several decades for sedation, its role in clinical practice has revealed broad variability in clinical response [
9-
11]; the sedation protocols and regimens varied among the studies. In the therapeutic dose range, DEX is not associated with respiratory depression despite deep sedation [
12,
13]. A loading dose of 1.0 ╬╝g/kg of body weight over 10 min on the label is recommended. A maintenance dose (0.4-0.7 ╬╝g/kg/h) is generally initiated and adjusted to maintain the target BIS. However, in this study, inappropriate sedation and airway obstruction were observed in some cases at the recommended dose.
The dose requirements for sedatives are decreased in elderly patients because of increased sensitivity to sedatives [
14]. Therefore, the usual dose for adults may induce over-sedation in elderly patients. Thus, we compared several dosing schedules of intravenous DEX in elderly patients receiving spinal anesthesia at therapeutic dose range.
A single dose of DEX (1.0 ╬╝g/kg of ABW) induced excessive sedation in about 47% of patients and the differences between the individual responses were large. Ramsay sedation score was highest around the 20-min period and then decreased afterwards [
15]. An optimal maintenance dose (0.2-0.25 ╬╝g/kg of ABW/h) of DEX after the loading dose (1.0 ╬╝g/kg of ABW) of DEX during spinal anesthesia has been reported in adult patients [
16,
17]. A single dose of DEX (1.0 ╬╝g/kg of ABW) takes 10.9 ┬▒ 1.2 min to reach BIS 80 in adults [
18]. An initial loading dose (1.0 ╬╝g/kg of ABW) followed by a maintenance dose (0.4-0.7 ╬╝g/kg of ABW/h) maintained BIS of 70-80 and required 10 min [
7] and 7.9 ┬▒ 4.0 min [
19], respectively, to reach BIS 80 in adults. In this study, mean BIS in all groups were maintained below BIS 65 from 30 min after the start of the initial loading dose to end of study period. Groups A and B had lower than adequate sedation levels from 30 min after the start of the initial loading dose to the end of the study period, but Group C maintained adequate sedation levels. Mean times required to reach BIS 80 were 6.1 ┬▒ 5.3 min, 5.0 ┬▒ 3.6 min, and 11.0 ┬▒ 8.6 min in groups A, B, and C, respectively. Median times required to reach BIS 80 in groups A and B were shorter than in the above-mentioned studies of adult patients [
7,
19]. Group C took longer than groups A and B to reach BIS 80. These are consistent with the findings that the dose requirements for sedatives are decreased in elderly patients [
11] and that higher doses induce faster sedation [
20].
The occurrence of bradycardia may be related to the administration of a loading dose. DEX-induced bradycardia resolved spontaneously or was readily treated by anticholinergics without adverse outcomes [
19]. In our study, the frequency of bradycardia was not statistically different among the groups.
There are several limitations to this study. First, the difference in side effects among the groups might not be observed in the statistical analysis because of lack of power, which is related to the small sample size of patients in each group. Further studies with larger sample sizes are required to confirm findings. Second, there were various types of surgical procedures. Body movement caused by the surgical procedure might interrupt the sedation. Third, we could not evaluate spinal anesthetic level and the effect of tourniquet pain as time passed. Over time, withdrawal of spinal anesthetic level might cause discomfort and pain and interfere with sedation. Fourth, the occurrence of hypotension could not be estimated due to the various types of procedures that induce bleeding and use a tourniquet. Fifth, because BIS constantly changes over time, it was hard to accurately measure it. These limitations should be considered when interpreting the results.
In conclusion, many other studies have reported that rapid administration or a high dose of DEX might produce side effects. Therefore, an initial loading dose of DEX that is 0.8 ╬╝g/kg of IBW administered for 10 min and then an infusion rate of less than 0.5 ╬╝g/kg of IBW/h are required for adequate sedation in elderly patients receiving spinal anesthesia.










