INTRODUCTION
The growing aging population and rising life expectancy have led to an increase in the prevalence of arthritis and, consequently, an increase in the number of total knee arthroplasty (TKA) procedures performed [
1]. TKA is scheduled commonly in the field of orthopedics. It has been reported that neuraxial blockade can reduce mortality and morbidity compared with general anesthesia in surgical patients with cardiac risk factors [
2]. Furthermore, epidural block, one of the most commonly used neuraxial blockades in TKA, is associated with a lower incidence of pneumonia, systemic infection, and other complications, compared with general anesthesia. In addition, epidural block has fewer respiratory side effects and is associated with less blood loss during surgery than general anesthesia. It is also conducive to pain management and rehabilitation after surgery [
3-
5].
Appropriate sedation can increase the quality of the epidural block, since awake patients may be nervous and fearful during surgery. Commonly used medications in an epidural block include midazolam, propofol, opioids, and dexmedetomidine. Zeyneloglu et al. [
6] reported that recovery time was longer after administration of dexmedetomidine compared with midazolam/fentanyl in relatively young patients. However, patients who undergo TKA are typically older, and because the effects of dexmedetomidine are hemodynamically stable, it can safely be used to induce sedation without respiratory depression, unlike other medications that can induce cardiorespiratory depression [
7-
9]. Although dexmedetomidine is safe with regard to hemodynamic stability and respiratory depression, inappropriate dosage increases recovery time [
10,
11]. Furthermore, the clearance rate of dexmedetomidine is decreased in elderly individuals; therefore, both side effects and cases requiring intervention during sedation are more frequent [
12].
The present study was performed to investigate the optimal dose of dexmedetomidine required for sedation during TKA with an operative duration longer than 120 min, as well as postoperative recovery.
MATERIALS AND METHODS
This randomized, prospective, controlled study was approved by the Institutional Review Board of our hospital (05-2013- 022). Sixty-three adult patients with an American Society of Anesthesiologists physical status I/II score who were scheduled for elective unilateral TKA were enrolled in the study. Informed consent was obtained for the administration of epidural anesthesia, sedation during surgery, and study participation. Patients with a history of arrhythmia; heart, renal, or liver failure; bleeding tendencies; or requirement for additional sedative were excluded.
Patients did not receive premedication. The following measurements were made upon arrival in the operating room: blood pressure (BP; non-invasive monitoring), heart rate (HR), arterial oxygen saturation (SpO2), and bispectral index score (BIS). The patient was placed in the lateral recumbent position, and an epidural catheter was positioned at the L3-L4 or L4-L5 level inserted 3 cm in situ. Subsequently, the patient was placed in the supine position, and oxygen was delivered at a rate of 3 L/min with a nasal cannula. Following a 3-ml test dose of 2% lidocaine with epinephrine (15 μg), a 10 ml dose of 0.75% bupivacaine and fentanyl (50 μg) was administered. After checking the level of epidural anesthesia, 1 μg/kg of dexmedetomidine was administered intravenously for 10 min as a loading dose. Atropine (0.5 mg) was administered intravenously if bradycardia (defined as HR less than 50 beats/min) was observed. Intravenous infusion of ephedrine (5 mg) with crystalloid (300 ml) was performed in response to hypotension, which was defined as a mean arterial pressure (MAP) of less than 60 mmHg. Prior to deflation of the tourniquet, fluid was infused as a precaution against hypotension.
Patients were divided into two groups: Group A (dexmedetomidine at a rate of 0.6 μg/kg/h) and Group B (dexmedetomidine at a rate of 1.0 μg/kg/h) (
Fig. 1). Two types of 50 ml preparations were administered intravenously as follows: Group A received 300 μg of dexmedetomidine in 47 ml of saline, and Group B received 500 μg of dexmedetomidine in 45 ml of saline. The syringes were prepared prior to anesthesia by nurses who did not participate in the study, and nurses in the operating room selected a sealed envelope randomly. The anesthesiologists and patients were blinded to group allocation.
Fig. 1
Flow chart. A patient in Group B was excluded.

MAP, SpO2, HR, and BIS were recorded at 10-min intervals. Intravenous administration of dexmedetomidine was stopped once the surgeon began to suture the surgical site. Patients were admitted to the postanesthesia care unit (PACU) after surgery; the assigned nurse recorded the modified Aldrete score (MAS), MAP, and BIS at 10-min intervals. The MAS included the following parameters: level of activity, respiration pattern, circulation (BP and HR), level of consciousness, and oxygen saturation. Patients were discharged from the PACU at an MAS of 10.
The primary endpoint was recovery time. Length of stay in the PACU was compared between the two groups. The effectiveness of sedation was evaluated based on the BIS.
All data were recorded as mean ± SD. StatView 5.0 (SAS Institute Inc., USA) was used for statistical analyses. The sample size was set at 27 to measure a 15-min delay in the PACU with a power of 80% (α < 0.05). The chi-squared test was used for categorical data and the Wilcoxon rank sum test was used for comparison of groups. A mixed effect model was used for repeated measures to identify differences in the BIS. Significance was defined as a P value of 0.05 or lower.
RESULTS
One patient in Group B who was awake and required additional sedative was excluded. The number of patients was thus 32 in Group A and 31 in Group B (
Fig. 1).
There were no significant differences between the two groups in terms of sex, age, weight, operation time, or amount of fluid administered during surgery (
Table 1).
Table 1
Patient Characteristic Variables of the Group
|
Group |
A (n = 32) |
B (n = 31) |
P value |
|
Sex (M/F) |
9/23 |
9/22 |
|
|
Age |
66.0 [58.3, 67.3] |
66.0 [55.0, 71.0] |
0.840 |
|
Weight |
61.0 [54.3, 72.7] |
60.0 [53.0, 70.0] |
0.570 |
|
Operation time (min) |
210.0 [191.3, 225.0] |
210.0 [180.0, 225.0] |
0.600 |
|
Plasma solution A (ml) |
1600.0 [1237.5, 1925.0] |
1500.0 [1350.0, 1975.0] |
0.720 |
|
Ephedrine |
7 (21.9) |
6 (19.4) |
0.403 |
|
Atropine |
5 (15.6) |
4 (12.9) |
0.380 |
Length of stay in the PACU was significantly longer in Group B (Group A: 53.3 ± 22.5 min, Group B: 71.5 ± 32.3 min, P = 0.01).
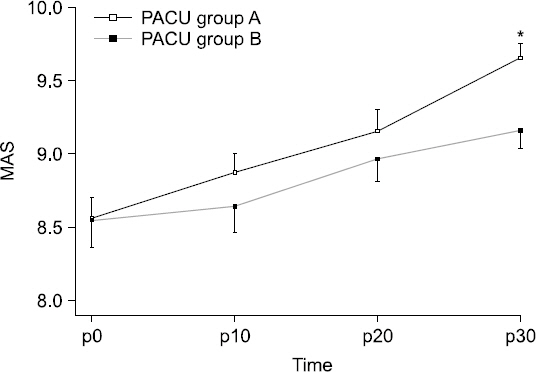
MAS was higher at 10, 20, and 30 min in Group A; however, a statistically significant difference was only observed at 30 min (P = 0.003,
Fig. 2).
Fig. 2
Postoperative changes (10 minute interval) of Modified Aldrelete Score (MAS) in postanesthesia care unit (PACU). Data are presented as mean ± SD. *P < 0.05 versus group B. A: loading dose followed by maintenance dose (0.6 μg/kg/h), B: loading dose followed by maintenance dose (1.0 μg/kg/h), MAS p0: MAS at the moment of PACU arrival.
There was no difference in the intravenous infusion time of dexmedetomidine (Group A: 208.5 ± 41.0 min, Group B: 203.7 ± 24.7 min, P = 0.58) or the time between arrival in the PACU and termination of the intravenous infusion (Group A: 35.9 ± 18.8 min, Group B: 35.5 ± 18.2 min, P = 0.92).
The HR and SpO
2 showed no significant differences. Although the BIS in the operating room was lower in Group B, the difference was not statistically significant except at 160 min (P = 0.042,
Fig. 3). The MAP in the operating room was not significantly different between the groups (
Fig. 4). The number of cases in which ephedrine was administered during surgery because of hypotension was seven in Group A and six in Group B. Atropine was administered owing to bradycardia in five cases in Group A and four cases in Group B. However, the MAP in the PACU was significantly lower in Group B than in Group A (
Fig. 4). Although the BIS in the PACU was higher in Group A than in Group B, the difference was not statistically significant.
Fig. 3
Changes (10 minute interval) of bispectral index (BIS) after intravenous infusion of the dexmedetomidine. Data are presented as mean ± SD. *P < 0.05 versus group B. A: loading dose followed by maintenance dose (0.6 μg/kg/h), B: loading dose followed by maintenance dose (1.0 μg/kg/h), BIS 0: bispectral index value of the dexmedetomidine infusion start time, BIS p0: bispectral index value at the moment of postanesthesia care unit arrival.

Fig. 4
Changes (10 minute interval) of mean arterial pressure (MAP) after intravenous infusion of the dexmedetomidine. Data are presented as mean ± SD. *P < 0.05 versus group B. A: loading dose followed by maintenance dose (0.6 μg/kg/h), B: loading dose followed by maintenance dose (1.0 μg/kg/h), MAP 0: mean arterial pressure of the dexmedetomidine infusion start time, MAP p0: mean arterial pressure at the moment of PACU arrival.

DISCUSSION
Dexmedetomidine, an alpha-2 adrenoreceptor agonist, has sedative and analgesic effects and has been introduced as a medication for sedation of patients receiving mechanical ventilation in the intensive care unit. Since dexmedetomidine can induce sedation without respiratory depression, its use as an adjuvant in regional anesthesia is increasing. However, there is a lack of research regarding the appropriate infusion dose of dexmedetomidine. Based on previous findings that the BIS level was generally above 70 and some patients awakened from sedation 100 min after injection with a dexmedetomidine maintenance dose of 0.2/0.4 μg/kg/h, a study was performed with dexmedetomidine maintenance doses of 0.6 and 1.0 μg/kg/h using an epidural block during TKA with a duration of 2 h or longer [
13].
The BIS was developed to evaluate the appropriateness of the operating environment by assessing the neurophysiological response to noxious stimuli during surgery, but it is not a criterion for the evaluation of sedation. However, objective and real-time testing is feasible, and sedation can be maintained without any interruption by external stimuli. Since the use of dexmedetomidine under epidural anesthesia is focused on reducing the response to external stimuli during surgery, the degree of sedation in the present study was evaluated using the BIS, and the BIS associated with an appropriate level of sedation was set at 60-80.
A BIS ≤ 80 was maintained in both groups by a 20-min dexmedetomidine infusion. The BIS showed no significant difference between the two groups except at 160 min after the start of infusion, when it was statistically significantly higher in Group A. Measurements after 160 min were not statistically significant because of the small number of patients. Therefore, an infusion dose of ≥ 0.6 μg/kg/h is required for sedation in regional anesthesia lasting longer than 2 h. Consistent with a report by Tufanogullari et al. [
14], both groups in the present study showed a high incidence of cardiovascular events (hypotension 7 [22%] and 6 [19%], bradycardia 5 [16%] and 4 [13%] in Group A and Group B, respectively) prompting the need for special precautions. Although it has been reported that recovery time is shortened when 0.2, 0.4, or 0.8 μg/kg/h of dexmedetomidine is used as an adjuvant to inhalational anesthetics, there is a dose-dependent association with the delay in recovery time [
14]. The BIS on arrival in the PACU is also associated with the dose of dexmedetomidine. According to Lee et al. [
15], the termination half-life of dexmedetomidine is 2-3 h, and the sedative effect is dose-dependent. We suggest that an infusion dose of ≥ 0.6 μg/kg/h after administration of the dexmedetomidine loading dose is required for a therapeutic effect that is greater than the termination half-life. Furthermore, when dexmedetomidine was administered as an adjuvant to inhalational anesthetics, because of the effect of the inhalational anesthetic, the effect on recovery time was different from that expected on the basis of the pharmacological features of dexmedetomidine alone. However, when dexmedetomidine is used as an adjuvant to epidural anesthesia, it is thought that the recovery is delayed in proportion to the dose because of the pharmacological features of dexmedetomidine. Similarly, because the effect of epidural anesthesia is greater on BP in the operating room than the effect of dexmedetomidine, there was no difference in MAP between the two groups in the present study, consistent with the findings of a previous study [
13]. However, in the PACU, the MAP differed between the two groups in proportion to the dexmedetomidine dose administered; the effect of dexmedetomidine alone persisted, and there was a negligible effect from the epidural anesthesia.
Usually patients who have undergone TKA have stayed in the PACU for 30 min. The length of stay was prolonged and the BIS levels were low upon arrival in the PACU, because, in the present study, the time at which the dexmedetomidine infusion was stopped was the time when the surgeon began suturing. To reduce the length of stay, it is likely better to stop the dexmedetomidine infusion when the main procedure is completed and immediately before removal of the tourniquet. The dexmedetomidine infusion was scheduled to begin after the start of epidural anesthesia. A patient’s anxiety may be reduced if the dexmedetomidine infusion is begun prior to epidural anesthesia. However, operation times can vary by surgeon, and thus in the present study, dexmedetomidine infusion was begun after epidural anesthesia was initiated.
The correlation between epidural anesthesia and changes in the BIS is still debatable. According to Sentürk et al. [
16], the BIS is not reduced by epidural anesthesia. However, according to Ishiyama et al. [
17], the BIS decreased when the patient was awake and under epidural anesthesia; yet, the reduction was with respect to the BIS levels in the awake state, and the BIS did not drop to levels as low as those during sedation (≤ 80). Therefore, the effect of epidural anesthesia itself on the BIS can largely be ignored.
A limitation of the present study is that there was not a large variation in the doses tested; for example, doses of 0.4 and 0.8 μg/kg/h were not included. However, as a dose of 0.4 μg/kg/h was found to be inappropriate for ≥ 2 h of sedation in a previous study [
13], we believe that the exclusion of this dose is justified.
Based on the findings of this study, in consideration of the delayed awakening or hemodynamic instability in the PACU, we suggest that 0.6 μg/kg/h is an appropriate dose of dexmedetomidine for sedation in surgeries involving ≥ 2 h of regional anesthesia. However, precautions should be taken, because this dose can often cause cardiovascular side effects.
ACKNOWLEDGEMENTS
This work was supported by Pusan National University Research Grant.
REFERENCES
2. Guay J, Choi P, Suresh S, Albert N, Kopp S, Pace NL. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2014; 1: CD010108PMID:
10.1002/14651858.CD010108.pub2.

3. Liu J, Ma C, Elkassabany N, Fleisher LA, Neuman MD. Neuraxial anesthesia decreases postoperative systemic infection risk compared with general anesthesia in knee arthroplasty. Anesth Analg 2013; 117: 1010-6. PMID:
10.1213/ANE.0b013e3182a1bf1c. PMID:
24023024. PMID:
PMC3800057.



4. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am 2013; 95: 193-9. PMID:
10.2106/JBJS.K.01682. PMID:
23269359.


5. Farag E, Dilger J, Brooks P, Tetzlaff JE. Epidural analgesia improves early rehabilitation after total knee replacement. J Clin Anesth 2005; 17: 281-5. PMID:
10.1016/j.jclinane.2004.08.008. PMID:
15950853.


6. Zeyneloglu P, Pirat A, Candan S, Kuyumcu S, Tekin I, Arslan G. Dexmedetomidine causes prolonged recovery when compared with midazolam/fentanyl combination in outpatient shock wave lithotripsy. Eur J Anaesthesiol 2008; 25: 961-7. PMID:
10.1017/S0265021508004699. PMID:
18538051.


7. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologistss. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002; 96: 1004-17. PMID:
10.1097/00000542-200204000-00031. PMID:
11964611.


8. Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg 2002; 95: 461-6. PMID:
10.1097/00000539-200208000-00042.


9. Park JW, Han JU, Shinn HK, Jung JK, Cha YD, Kang SA, et al. The effect of intravenous dexmedetomidine on the duration of brachial plexus block. Anesth Pain Med 2012; 7: 307-11.
10. Phelps JR, Russell A, Lupa MC, McNaull P, Pittenger S, Ricketts K, et al. High-dose dexmedetomidine for noninvasive pediatric procedural sedation and discharge readiness. Paediatr Anaesth 2015; 25: 877-82. PMID:
10.1111/pan.12569. PMID:
25565076.


11. Makary L, Vornik V, Finn R, Lenkovsky F, McClelland AL, Thurmon J, et al. Prolonged recovery associated with dexmedetomidine when used as a sole sedative agent in office-based oral and maxillofacial surgery procedures. J Oral Maxillofac Surg 2010; 68: 386-91. PMID:
10.1016/j.joms.2009.09.107. PMID:
20116712.


12. Kuang Y, Zhang RR, Pei Q, Tan HY, Guo CX, Huang J, et al. Pharmacokinetic and pharmacodynamic study of dexmedetomidine in elderly patients during spinal anesthesia. Int J Clin Pharmacol Ther 2015; 53: 1005-14. PMID:
10.5414/CP202443. PMID:
26413731.


13. Ok HG, Baek SH, Baik SW, Kim HK, Shin SW, Kim KH. Optimal dose of dexmedetomidine for sedation during spinal anesthesia. Korean J Anesthesiol 2013; 64: 426-31. PMID:
10.4097/kjae.2013.64.5.426. PMID:
23741565. PMID:
PMC3668104.



14. Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg 2008; 106: 1741-8. PMID:
10.1213/ane.0b013e318172c47c. PMID:
18499604.


15. Lee S, Kim BH, Lim K, Stalker D, Wisemandle W, Shin SG, et al. Pharmacokinetics and pharmacodynamics of intravenous dexmedetomidine in healthy Korean subjects. J Clin Pharm Ther 2012; 37: 698-703. PMID:
10.1111/j.1365-2710.2012.01357.x. PMID:
22650799.


16. Sentürk M, Güçyetmez B, Ozkan-Seyhan T, Karadeniz M, Dinçer S, Akpir D, et al. Comparison of the effects of thoracic and lumbar epidural anaesthesia on induction and maintenance doses of propofol during total i.v anaesthesia. Br J Anaesth 2008; 101: 255-60. PMID:
10.1093/bja/aen160. PMID:
18534970.


17. Ishiyama T, Kashimoto S, Oguchi T, Yamaguchi T, Okuyama K, Kumazawa T. Epidural ropivacaine anesthesia decreases the bispectral index during the awake phase and sevoflurane general anesthesia. Anesth Analg 2005; 100: 728-32. PMID:
10.1213/01.ANE.0000159868.06847.47. PMID:
15728060.











