INTRODUCTION
Reducing the incidence of postoperative complications and mortality continues to be an area that warrants improvement through research. Postoperative complication risk is determined by numerous factors including a patientŌĆÖs underlying medical condition, type of surgery required, and anesthetic management.
Immediate postoperative care in an intensive care unit (ICU) can be crucial in patients with a high rate of perioperative morbidity. Reports indicate that immediate postoperative ICU admission in high-risk patients can reduce the rate of postoperative complications and mortality [
1]. There is however, a limit to the number of patients that can be admitted to the ICU after surgery due to a limitation in ICU resources. Most postoperative ICU admissions for patients with a high risk surgical procedure was planned regardless of intraoperative status. Consequently, risk stratification of patients undergoing surgery is seen as mandatory.
The surgical Apgar score (SAS) is a simple tool used for intraoperative risk stratification. The SAS is a score of 10 points calculated based on the estimated blood loss, lowest heart rate, and lowest mean arterial blood pressure during surgery [
2]. There are numerous reports that SAS can predict postoperative mortality and morbidity in many surgical areas [
2-
4].
However, there is a lack of research about the relationship between post-operative outcome and SAS for patients who are admitted to the ICU immediately after surgery. The purpose of this study was to evaluate this relationship by observing the SAS and its associated postoperative outcomes. Factors such as postoperative complications, duration of ICU stays, and patient mortality following surgery were used to evaluate the patientŌĆÖs status.
MATERIALS AND METHODS
This study was performed retrospectively in patients who were admitted to the ICU immediately after undergoing surgery involving general anesthesia. The study population included patients who underwent surgery between January 2017 and August 2017, with a six-month follow-up. We excluded patients who underwent open-heart surgery, transplantation, endovascular procedures, or reoperation.
General anesthesia was induced according to our hospitalŌĆÖs standards, with propofol (2 mg/kg) or etomidate (0.2 mg/kg), and muscle relaxation with rocuronium bromide (0.6-1 mg/kg). Anesthesia was maintained by continuous infusion with remifentanil (0.05-0.15 ╬╝g/kg/min) and inhalation of desflurane, according to the bispectral index (40-60). After the operation, the patients were transferred to the surgical or trauma ICU and received intensive care by one or two intensivists.
Anesthetic monitoring involved various methods including electrocardiography, plethysmography, and blood pressure measurement (invasive or noninvasive methods). Blood pressure was controlled to ┬▒ 20% of baseline during the surgery. Hypotension beyond this range was treated with adequate vasopressor and fluid administration while hypertension was treated by increasing the anesthetic agent and administering a vasodilator. If hypotension persisted following first line treatment protocols, then a vasopressor was administered by continuous rate infusion. Anesthetic records were used to investigate which medications were administered to each patient. Patients with severe tachycardia (heart rate [HR] > 120 beats/min) or bradycardia (HR < 45 beats/min) without a known reason were treated with appropriate medication. Data was not included for the 30 min following drug administration.
Intraoperative SAS was calculated from the lowest heart rate, lowest mean blood pressure, and estimated blood loss during surgery. These values were taken from the anesthetic record and are shown in
Table 1 [
2]. Additionally, using the data acquisition algorithm derived by Regenbogen et al. [
5], we excluded the extraphysiologic values for HR (less than 20 or greater than 200 beats/min) and MAP (less than 25 or greater than 180 mmHg). We then divided the study subjects into three groups according to their SAS score: 7-10, 5-6, and Ōēż 4. This was due to the relatively low number of patients with high scores.
Table┬Ā1
|
0 |
1 |
2 |
3 |
4 |
|
EBL (ml) |
> 1,000 |
601-1,000 |
101-600 |
< 100 |
|
|
Lowest MAP (mmHg) |
< 40 |
40-54 |
55-69 |
Ōēź 70 |
|
|
Lowest HR (beats/min) |
> 85 |
76-85 |
66-75 |
56-65 |
< 55 |
Data from both anesthetic records and medical records were collected from the electronic hospital chart system. Information collated included patient age, gender, and body mass index. The type of surgery performed, emergency status, anesthesia duration, ejection fraction, co-morbidities (such as diabetes, hypertension, coronary artery disease, and chronic obstructive pulmonary disease), and American Society of Anesthesiologists-physical status (PS) were also recorded. In addition, ICU electronic medical records were used to collect data such as ICU stay period, mechanical ventilation requirement, continuous renal replacement therapy (CRRT) requirement, use of inotropes, and transfusion therapy.
Major postoperative complications were investigated for 30 days after the surgery. Major complications included: myocardial infarction; pneumonia; pulmonary embolism; stroke; acute renal failure; cardiac arrest requiring cardiopulmonary resuscitation; coma for Ōēź 24 h; bleeding requiring Ōēź 4 units of red blood transfusion within 72 h of the operation; wound disruption; deep or organ-space surgical infection; sepsis; septic shock; systemic inflammatory response syndrome; deep vein thrombosis; unplanned intubation; ventilator use for > 48 h; vascular graft failure; death [
6].
Variation between the preoperative status and clinical outcome in the groups was evaluated using the PearsonŌĆÖs Žć2 or ANOVA where appropriate. The regression between SAS and various postoperative characteristics, such as ICU stay, was also evaluated. Univariate and multivariate logistic regression models were used to evaluate which of the variables influenced postoperative complications and mortality. ICU characteristics such as duration of patient stay were considered to correspond directly to postoperative complications and mortality; however, the multivariate logistic regression was conducted with variables measured during surgery. Statistical analysis was conducted using the Sigmastat┬« program (version 4.0, SYSTAT system, USA) and considered significant at a P value of < 0.05.
RESULTS
During the study period, a total of 669 patients were admitted to the ICU after surgery, and 543 patients were included in this study excluding patients with organ transplantation, open heart surgery, and two or more surgeries. There were 173 patients (110 males and 63 females) who underwent emergency operations, and 370 patients (216 males and 154 females) who underwent elective procedures. The most common reason for postoperative admission to the ICU was to allow more vigilant patient monitoring. This was planned for 87.5% of patients in the study population.
In the studied population, the most common surgery was a hepatobiliary operation (161 patients, 29.7%). The mean age of the patients was 60.5 (┬▒ 15.6) years and the mean SAS was 5.4 (┬▒ 2.0). The mean ICU stay period was 4.1 (┬▒ 6.9) days and the period from surgery to discharge was 26.2 (┬▒ 29.1) days. One hundred and sixty patients (29.5%) had postoperative complications and 34 patients (6.3%) died.
A summary of the assigned groups according to the SAS is presented in
Table 2. It can be seen that the group with the lowest SAS (0-4) was of significantly younger age than the other groups. As the SAS decreased, the amount of intraoperative infusion of crystalloid, colloid, and packed red blood cells increased. The frequency of major complications during the 30-day period following surgery was 15.7% (28 cases), 27.8% (54 cases), and 45.6% (78 cases) from the highest to lowest SAS groups. The mortality rate was found to increase with a decreasing SAS, with values of 2.2%, 5.2%, and 11.7%. The group with an SAS score below 4 was also shown to have a higher likelihood of emergency and trauma surgery.
Table┬Ā2
Characteristics of the Patient, Intraoperative, and ICU According to Surgical Apgar Score (SAS)
|
Variable |
Group A |
Group B |
Group C |
P value |
|
|
|
|
SAS Ōēź 7 (n = 178) |
SAS 5-6 (n = 194) |
SAS Ōēż 4 (n = 171) |
|
Sex, M/F |
100/78 |
113/81 |
111/60 |
0.216 |
|
Age (yr) |
63.0 (53.0, 73.0) |
64.0 (52.5, 74.0) |
58.5 (49.8, 69.0)*,ŌĆĀ
|
0.003 |
|
BMI (kg/m2) |
23.0 (21.3, 25.3) |
23.2 (20.9, 25.7) |
23.4 (21.3, 25.2) |
0.957 |
|
Emergency OP |
23 (12.9) |
54 (27.8)*
|
96 (56.1)*
|
< 0.001 |
|
DM |
35 (19.7) |
39 (20.1) |
35 (20.5) |
0.982 |
|
HTN |
70 (39.3) |
81 (41.8) |
63 (36.8) |
0.795 |
|
Comorbidity > 1 |
67 (39.0) |
58 (33.7) |
47 (27.3) |
0.103 |
|
Trauma |
7 (3.9) |
17 (8.8)*
|
39 (22.8) |
< 0.001 |
|
EF (%) |
65.0 (61.0, 69.0) |
65.0 (60.0, 69.0) |
65.0 (58.0, 68.0) |
0.149 |
|
Hepatobiliary OP |
37 (20.8) |
58 (29.9)*
|
66 (38.6)*,ŌĆĀ
|
0.001 |
|
Brain OP |
33 (18.5) |
36 (18.6) |
39 (22.8) |
0.522 |
|
Lung OP |
48 (27.0) |
23 (11.9)*
|
9 (5.3)*,ŌĆĀ
|
< 0.001 |
|
Other |
60 (33.7) |
77 (39.7) |
57 (33.3) |
0.333 |
|
Crystalloid (ml) |
1,425.0 (687.5, 2,600.0) |
2,400.0 (1,075.0, 4,100.0)*
|
3,400.0 (2,237.5, 4,825.0)*,ŌĆĀ
|
< 0.001 |
|
Colloid (ml) |
500.0 (487.5, 500.0) |
500.0 (500.0, 975.0)*
|
700.0 (500.0, 1,000.0)*,ŌĆĀ
|
< 0.001 |
|
RBC transfusion at OR (U) |
0.0 (0.0, 0.0) |
0.0 (0.0, 2.0)*
|
2.0 (0.0, 5.0)*,ŌĆĀ
|
< 0.001 |
|
Postoperative characteristics |
|
ŌĆāComplication Ōēź 1 |
28 (15.7) |
54 (27.8)*
|
78 (45.6)*,ŌĆĀ
|
< 0.001 |
|
ŌĆāMortality |
4 (2.2) |
10 (5.2)*
|
20 (11.7)*,ŌĆĀ
|
< 0.001 |
|
ŌĆāICU readmission |
4 (2.2) |
8 (4.1) |
10 (5.8) |
0.233 |
|
ŌĆāICU stay (d) |
1.0 (1.0, 2.0) |
1.0 (1.0, 3.0)*
|
3.0 (1.0, 9.0)*,ŌĆĀ
|
< 0.001 |
|
ŌĆāAPACHE score |
13.1 ┬▒ 6.4 |
12.7 ┬▒ 6.7 |
15.7 ┬▒ 8.8 |
0.136 |
|
ŌĆāPacked RBC transfusion at ICU (U) |
0.0 (0.0, 0.0) |
0.0 (0.0, 0.0) |
0.0 (0.0, 2.0)*
|
< 0.001 |
|
ŌĆāVentilator use |
8 (4.5) |
30 (15.5)*
|
67 (39.2)*,ŌĆĀ
|
< 0.001 |
|
ŌĆāInotropic use |
4 (2.2) |
19 (9.8)*
|
41 (24.0)*,ŌĆĀ
|
< 0.001 |
|
ŌĆāCRRT use |
0 (0) |
6 (3.1)*
|
9 (5.3)*,ŌĆĀ
|
0.011 |
|
ŌĆāDischarge from OP (d) |
12.0 (8.8, 17.0) |
17.0 (11.0, 27.0)*
|
24.0 (12.0, 51.0)*,ŌĆĀ
|
< 0.001 |
Patients with a SAS score lower than 4 also experienced a longer duration ICU stay with increased requirement for mechanical ventilation and inotropic administration. The duration from time of surgery to patient discharge was also longer with a lower SAS score. The SAS score had a weak negative correlation with the duration of ICU stay (R = 0.307, P < 0.001) and postoperative day of discharge (R = 0.290, P < 0.001) (
Fig. 1). In the receiver operating characteristic (ROC) curve analysis between SAS and the postoperative complication rate, the area under curve was 0.663 (95% confidence interval [95% CI] 0.621-0.702, P < 0.001) and the discrimination score was 4 (sensitivity 47.48 [39.0-56.1], specificity 73.95 [69.4-78.2], + likelihood ratio [LR]/-LR 1.82/0.71) (
Fig. 2).
Fig.┬Ā1
Correlation between surgical Apgar score (SAS) and intensive care unit (ICU) stay (A), and discharge day after surgery (B). In (A), there is a weak negative correlation between the duration of ICU stay and SAS (R = 0.307, P < 0.001). In (B), there is also a weak negative correlation between hospital stay and SAS (R = 0.290, P < 0.001). Regr: regression.
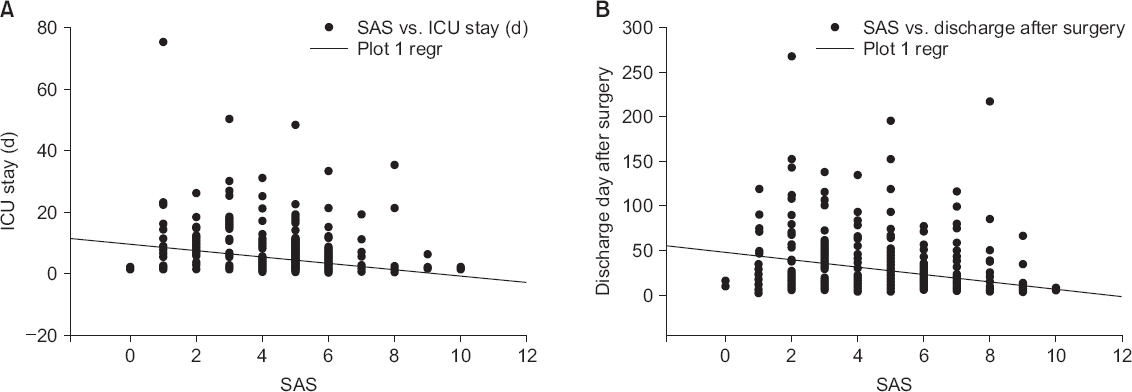
Fig.┬Ā2
Receiver operating characteristic curve showing the performance of surgical Apgar score to predict postoperative mortality. Area under curve (AUC) (95% confidence interval) is 0.663 (0.621-0.702).

For every point score increase in SAS, the univariate odds ratio of postoperative complications or mortality increased by 40% (odds ratio [OR] 0.688, 95% CI 0.621-0.762, P < 0.001; OR 0.634, 95% CI 0.532-0.756, P < 0.001, respectively). When analyzing this score by the three SAS groups, according to univariate logistic regression, postoperative complications and mortality rates doubled when compared to the reference group (SAS 7-10) (OR 2.097, 95% CI 1.637-2.684, P < 0.001; OR 2.287, 95% CI 1.419-3.686, P < 0.001, respectively). In univariate regression, the SAS, operation time, use of inotrope, and mechanical ventilation were found to be correlated. However, in multivariate logistic regression, major postoperative complications were not found to be related to the SAS score, operation time, use of inotrope, or mechanical ventilation during the surgery.
DISCUSSION
This study showed that, of the patients admitted to ICU immediately after surgery, those with a low SAS were of a younger age, were more likely to have undergone emergency surgery, and had an increased likelihood of fluid and packed red blood cell infusions during surgery. A low SAS was also found to be associated with a longer ICU stay, a higher requirement for inotropes, and mechanical ventilation, and a higher risk of a major postoperative complication. In summary, a low SAS during surgery, despite postoperative ICU care, is directly proportional to a higher possibility of the incidence of a major postoperative complication or death. Many patients who undergo surgical procedures are exposed to stressful conditions, which can increase the risk of mortality and morbidity in the perioperative period. Although the incidence is relatively low due to ongoing improvements in surgical procedures and anesthetic management, the overall rate of complications continues to be higher in high-risk patients or high-risk surgeries. Therefore, it is important to recognize and closely monitor high-risk patients during the perioperative period. Identification of these patients coupled with appropriate intervention is increasingly becoming an important preoperative requirement.
To stratify the risk of surgical patients, a number of scoring systems have been developed to better evaluate perioperative morbidity, and therefore, the likely postoperative outcome. Examples include American Society of Anesthesiologists-PS, Physiologic and Operative Severity Score for the Enumeration of Mortality and Morbidity (POSSUM), Revised Cardiac Risk Index (RCRI), and Acute Physiology and Chronic Health Evaluation (APACHE) [
7-
10].
American Society of Anesthesiologists-PS assigns a score of I-V based on the preoperative physiological state of patients and can therefore be easily measured [
7,
11]. American Society of Anesthesiologists-PS has some usefulness in predicting postoperative mortality; however, this score does not include surgical risk, and inter-user variability can be present within the system [
11,
12]. POSSUM is calculated based on 12 physiologic and 6 intraoperative variables. A modified calculation method is generally applied to lower risk groups, such as colorectal surgery, to limit over-prediction with some diseases [
8,
13]. Several studies have reported consistent accuracy with the use of this calculation method [
13,
14]. The RCRI is mainly used to predict major postoperative cardiac complications in patients undergoing non-cardiac surgery [
15,
16]. It incorporates six components: recent high-risk coronary artery disease, history of heart failure, history of stroke, renal insufficiency, diabetes mellitus, and high-risk surgical procedures [
9]. Although this index can discriminate the cardiac risk of individual patients, there is a limitation in that the absolute risk of cardiac complications cannot be accurately predicted [
17]. It is therefore necessary to add in another biochemical test, such as brain natriuretic peptide (BNP), N-terminal-proBNP, or Troponin, to more accurately classify the overall patient risk [
16,
17]. The APACHE II score is designed for use in the ICU [
10] and to evaluate disease severity by measuring 12 acute physiologic factors in addition to age, chronic health, and the Glasgow coma scale. However, it is of limited use in daily practice as it requires too many parameters, and it does not take into account the presenting comorbidities of the patient [
10,
18].
These scoring systems have been reported to help clinicians identify patients who are at a high risk of postoperative complications [
13]. However, most of these scoring systems are complex and calculated with many variables. Resultantly, they can be difficult to apply in situ. A new scoring system, which can be applied immediately and measured during surgery, has been proposed. In 2007, Gawande et al. [
2] proposed a scoring system that predicts postoperative complications calculated from only three variables: lowest heart rate, lowest mean arterial pressure, and estimated blood loss measured during surgery. The score calculation method for each variable is shown in
Table 1. The patient is assigned a score out of 10, similar to the widely used obstetrical Apgar score.
Low score groups have a high risk of postoperative complications while a high score indicates a low risk. It has been shown in general and vascular surgeries that SAS is closely related to postoperative morbidity and 30-day mortality [
2]. This scoring system, when applied to specific surgical fields, has proven to be effective in predicting postoperative morbidity [
3,
4]. There was also a report that the SAS in patients undergoing neurosurgical surgery accurately predicted patient mortality and postoperative complication rates within 30 days of surgery as well as extended stays in the ICU and hospital [
19]. These authors suggested that the ability to calculate this score in real time during surgery could then allow the neurosurgical team to direct more appropriate postoperative treatment to patients at higher risk of complications or mortality [
19]. Kinoshita et al. [
20] reported that the postoperative mortality increases by 3.65 times for each 2 point decrease in SAS score. The study population involved patients who underwent surgery with general or regional anesthesia over a 4-year period. Therefore, this score is considered to be one of the most useful tools used to assess how intraoperative patient management can affect postoperative outcome. There were, however, other studies showing contrasting results. Urrutia et al. [
21] reported that patients who underwent general orthopedic surgery did not have an increase in the relative risk of postoperative complications with a declining SAS. With spinal surgery however, the SAS was a more accurate predictor when patients were divided into subgroups.
Unlike American Society of Anesthesiologists-PS, the SAS is focused only on the intraoperative status of the patient and does not incorporate the patientŌĆÖs condition preoperatively. However, it has been reported that the SAS correlates with a patientŌĆÖs preoperative status, such as pre-existing comorbidities and operative complexity, and can therefore, effectively identify patients with a high risk of postoperative complication [
22].
The first 48 h after surgery is a critical period for high risk patients, and planned admission to the ICU can further reduce the postoperative mortality rate [
1]. In the present study, patients were not assigned to the ICU based on this score. In a retrospective study of 8,501 patients, 8.7% of patients were admitted to the ICU immediately after surgery. A multivariate adjusting model of these patients showed a strong correlation between the SAS score and the decision to admit a patient to the ICU [
23]. Patients with a SAS of two points or less were fourteen times more likely to be admitted to the ICU, when compared with the reference group of patients with a SAS of 7-8 [
23]. Stoll et al. [
24] reported that high SAS was not correlated with duration of ICU stay, but with the decrease rate of ICU admission. Therefore, in addition to various other risk scoring systems, the SAS may be used as an important factor in determining the requirement for ICU admission after surgery.
The intention of this study was to evaluate the relationship between SAS and postoperative complications and identify characteristics of those patients admitted to the ICU for various reasons such as surgery complexity, preoperative comorbidities, or vigilant monitoring. Patients with low scores were more likely to have undergone emergency surgery and were more likely to have required fluid and blood product administration during surgery. Patients with low scores were also more likely to have an increased duration of stay, and use mechanical ventilation, inotropes, and CRRT in the ICU. In addition, the incidence of postoperative complications, including mortality, was higher in the lower SAS group.
In this study, with a one-point increase in SAS score, postoperative complications and mortality decreased by 40%. Risk was doubled with each lower level SAS group. Therefore, it can be considered that this score, which is measured during surgery, corresponds closely to an increased risk of postoperative complication. Consequently, more vigilant monitoring and adequate intervention in the immediate postoperative period are vital for the patient who is admitted to the ICU, especially those with a low SAS score.
Some of the limitations of this study are as follows: firstly, this study was retrospective, and further studies determining real time admission requirements to the ICU, based on this score, are necessary; secondly, evaluation of the major postoperative complications cannot disregard the preand intra-operative management as well as post-operative ICU management. However, patients transferred to the ICU after the surgery received hospital-based standard care by two intensivists. Therefore, it is thought that the difference in postoperative ICU care in patients would not have been significant. Moreover, we did not evaluate the relationship with various other scoring systems in the patient groups admitted to the ICU. This study also has some problems with respect to patientŌĆÖ baseline demographics. When examining the characteristics of the study participants, younger patients, and those with a hepatobiliary operation or emergency operation had a low SAS. In addition, trauma patients were younger than lung surgery patients. It may be thought that complication and mortality is correlated with trauma and not with a low SAS. Lastly, there may be some limitations in expanding the results of this study to other patients because it was a study of patients who were admitted to the ICU after surgery for various reasons.
The results of this study suggest that patients with a low SAS on postoperative admission to the ICU have an increased risk of postoperative complication and mortality. Therefore, by communicating accurate intra-operative patient information to the ICU, the quality of patient treatment can be greatly improved.











