Ultrasound-guided placement of a permanent peripheral nerve stimulator in a patient with complex regional pain syndrome -A case report-
Article information
Abstract
A 56-year-old man complained of continuous pain in the right foot that began 6 months after undergoing surgery on the right calcaneus bone. The patient was diagnosed with complex regional pain syndrome (CRPS) type I and was treated with medication, lumbar sympathetic ganglion blocks, epidural nerve blocks, and spinal cord stimulation. However, all treatments were halted because they were ineffective or complications developed. Peripheral nerve stimulation (PNS) was planned after confirming the analgesic effects of a sciatic nerve block, and the patient received PNS via minimally invasive ultrasound-guided electrode placement. PNS reduced the pain intensity and the incidence of paroxysmal pain. Other than discomfort at the battery insertion site (resolved with re-implantation), the patient developed no complications. These results suggest that ultrasound-guided minimally invasive PNS is a safe and effective treatment for patients with CRPS in the lower extremities.
INTRODUCTION
Complex regional pain syndrome (CRPS) can occur in patients with a history of trauma, nerve damage, fracture, stroke, and spinal surgery. It is a neuropathic disorder with diverse clinical symptoms, including spontaneous pain, allodynia, hyperalgesia, and autonomic and motor neuron dysfunction in diverse peripheral areas [1,2]. Proposed mechanisms of CRPS include trauma-induced cytokine release, neurogenic inflammation, sympathetically maintained pain, and central sensitization due to chronic pain [2,3]. Understanding these underlying mechanisms is essential in order to apply multiple therapeutic methods for patients with CRPS [4]. However, there is still no definitive treatment for CRPS despite advances in therapeutic methods such as medications, nerve blocks, and spinal cord stimulation (SCS) [1].
Unlike SCS, only a few reports have investigated the role of peripheral nerve stimulation (PNS) in CRPS. Although the underlying mechanism of how PNS reduces pain has not been identified, evidence indicates that the analgesic effects are related to interactions within multiple regions of the nervous system. PNS may reduce pain by inhibiting abnormal pain perception by Aβ nerve fibers and may also have a sympatholytic effect via α-1 receptors in the peripheral and central nervous systems [5].
We report successful pain management in a patient with severe CRPS using PNS at the sciatic nerve after ultrasound-guided minimally invasive electrode placement.
CASE REPORT
Written consent for this case report was obtained from the patient. A 56-year-old man complained of continuous pain in the right foot that began 6 months after undergoing surgery (internal fixation due to right calcaneus bone fracture). The patient was suffering from hyperalgesia, allodynia, spontaneous pain, sweating, edema, cold sensation, and color changes in the right foot and ankle. The patient described the pain as severely sharp and electric, and scored the pain intensity as 7–8/10 using a visual analog scale (VAS). Under the impression of CRPS type I, we first prescribed oral medications (gabapentin 3,600 mg, tramadol mixture 150 mg, and nortriptyline 25 mg/day). However, the medications were not effective, and the patient underwent a lumbar sympathetic ganglion block (radiofrequency thermocoagulation and alcohol) with an epidural nerve block. The pain intensity was successfully reduced to a VAS score of 2–3/10.
The patient also received ankle arthrodesis to further reduce pain during ambulation. Unexpectedly, the VAS increased to 7/10 postoperatively, and the preoperative treatment methods were no longer effective. The patient was hospitalized and epidural pain management (2 ml/h 0.5% lidocaine and 2 mg/day morphine) effectively reduced pain intensity to 2/10 (VAS). However, the patient began to complain of back pain, fever, hypoxemia, and delirium 1 week later. The patient was diagnosed with an epidural abscess in the lumbar region (L1–4) via magnetic resonance imaging and was treated in the intensive care unit for 2 months due to the development of acute respiratory distress syndrome.
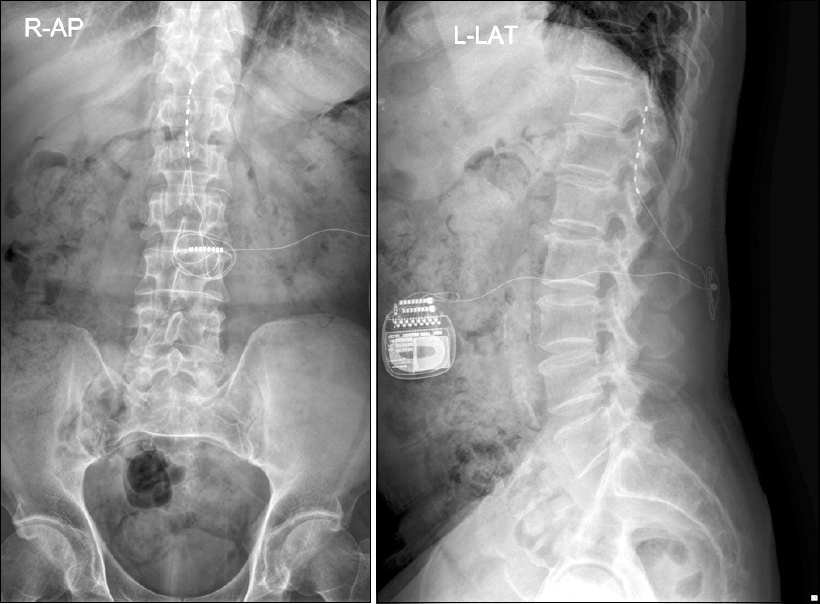
Next, we performed SCS after the patient recovered fully. The VAS decreased from 8/10 to 2/10 with temporal SCS, but the treatment was stopped 5 days later due to voiding difficulty and pneumonia. A permanent SCS was placed 3 months later, after confirming analgesic effects without complications with another temporal SCS (Fig. 1). Unfortunately, stimulation to the heel region (most painful region) only lasted for 1 week, and we were unable to stimulate the heel region through repositioning and electrode replacement. It is possible that the previous epidural abscess at the stimulation site affected the nerve stimulation.
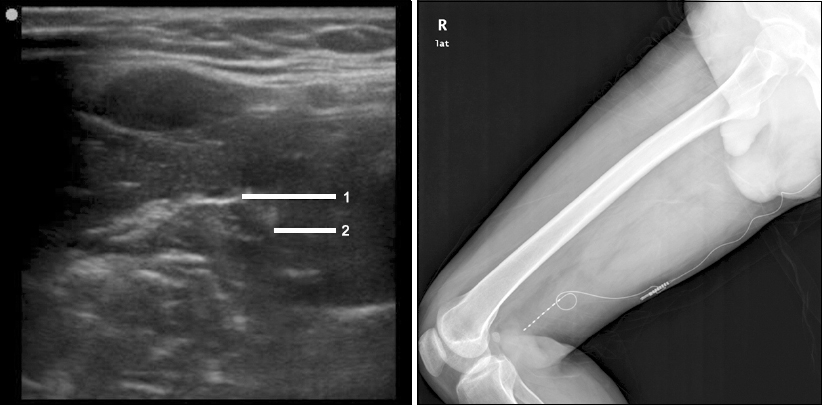
We then scheduled PNS after an extensive discussion with the patient. A proximal sciatic nerve block was performed prior to PNS to predict the analgesic effects. Levobupivacaine (5 ml of 0.375%) induced sensory depression and pain reduction in the right foot. For the procedure, the patient was placed in the prone position and the procedure was performed at the mid-thigh level, where there is the least movement. The gluteal and thigh region was sterilized, and local anesthetics were injected at the mid-thigh level before targeting the sciatic nerve. The sciatic nerve was identified between the biceps femoris muscle and semitendinosus and semimembranosus muscles. A 14 G needle was placed adjacent to the sciatic nerve under ultrasonographic guidance (in-plane technique). To confirm proper positioning and facilitate electrode placement, we first injected 5% D/W under ultrasonography. Fluids containing electrolytes were not used to avoid the effects of electrical conductivity. The proper electrode position (8 polar electrode lead, Medtronic, Minneapolis, MN, USA) was determined by confirming accurate stimulation by the patient. A subcutaneous incision was made to fixate the lead to the biceps femoris muscle fascia, and a pulse generator was connected after subcutaneous tunneling (Fig. 2). Intensity settings ranged from 0.6 to 5 V to obtain stimulation of 1.4–10.4 mA. Such stimulation induced only paresthesia and no muscle contractions. During the trial PNS (1 week), pain subsided from 8/10 to 2/10 (VAS), and the allodynia disappeared. A RestoreUltra (Medtronic) rechargeable generator was implanted in the right gluteal region. Other than discomfort at the battery insertion site, which was resolved by re-implantation, the patient has not suffered from any complications during the last 4 years. Pain intensity has decreased consistently to 2/10 (VAS), and the incidence of paroxysmal pain has been reduced to 70%.
DISCUSSION
Prompt diagnosis and treatment are essential for a good prognosis in patients with CRPS, and diverse treatment modalities must focus on reducing pain and promoting functional recovery [4]. Various treatment methods, such as medications, nerve blocks, physiotherapy, psychotherapy, and nerve stimulation, have been introduced, but there is still no definitive treatment for CRPS. Although several studies show conflicting results, SCS is now widely acknowledged to reduce pain and improve sleep quality in patients with CRPS. Recent advances in neurostimulator devices and medical techniques have also made SCS a cost-efficient treatment modality [6]. However, in our case, SCS was not only unsuccessful, but also caused voiding difficulty. We believe that fibrosis and adhesions from the previous epidural abscess and meningitis affected the SCS. The difficulty voiding during SCS may have been caused by changes in the autonomic nervous system, the poor general condition due to pneumonia, and changes in the blood concentration of antidepressants.
Unlike SCS, PNS has not received much attention in CRPS. Mirone et al. [7] reported successful PNS of the median nerve for CRPS type II after multiple carpal tunnel surgery. PNS is now regarded as a valid treatment option for iatrogenic type of the CRPS [5,7]. Hassenbusch et al. [8] analyzed the effect of permanent PNS in 30 patients with CRPS and suggested that PNS provides good relief in cases where symptoms are limited to a single major nerve. Huntoon and Burgher [9] also reported that minimally invasive ultrasound-guidance to implant PNS permanently was effective in six of eight (75%) patients with untreatable neuropathic pain. Such studies suggest that permanent PNS is effective for treating nerve injury-induced neuropathic pain, CRPS type II, and CRPS symptoms localized to one major nerve. Although the patient in our case was diagnosed with CRPS type I, PNS was considered a treatment option because the pain was limited to the sciatic nerve dermatome and a sciatic nerve block was effective. Our case suggests that permanent PNS should be considered in patients with CRPS with neuropathic pain limited to a single major nerve after confirming sufficient analgesic effects of a nerve block with local anesthetics.
Ultrasonography has become a valuable tool in pain medicine and advances in ultrasonographic visualization have significantly improved the outcomes of peripheral nerve interventions [10]. Although ultrasonography is useful for placing a surgical nerve stimulator, the biggest advantage arises from ultrasound-guided minimally invasive procedures. This approach is particularly useful for trial PNS, as electrodes can be removed without a surgical incision if the results are negative, making it possible to avoid increased pain from an incision. Other complications that can occur after surgical placement of an electrode include injury from nerve dissection, perineural tissue scarring, nerve ischemia due to tight fixation of the flat electrode, and a reaction to the implanted material. Another advantage of ultrasound-guided minimally invasive procedures is the ability to assess proper electrode position in real time. In our case, we were also able to successfully place an electrode at the sciatic nerve minimally invasively with the aid of ultrasonography without any complications.
PNS has a lower success rate when applied to the low extremities. Mobbs et al. [11] reported that the success rate of PNS is significantly lower in the lower extremities when compared with the upper extremities (65% vs. 24%). Possible mechanisms underlying the low success rate are difficulties fixing the electrode due to weight bearing and difficulties constantly stimulating sensory fibers deep within the sciatic nerve [11]. Thus, we placed the electrodes parallel to the sciatic nerve using the in-plane technique under ultrasonographic guidance to increase the contact surface between the nerve and electrodes. The lead was fixed at the mid-thigh level, where there is less motion. We also believe that our active interaction with the patient during the procedure facilitated proper electrode placement.
In conclusion, permanent PNS is an effective treatment method for patients with CRPS who do not respond to supportive treatment and are unable to perform or do not respond to SCS. Ultrasound-guided minimally invasive placement of a PNS electrode may improve outcomes by reducing complications associated with open surgery.