Pharyngeal reperforation following incentive spirometry - A case report -
Article information
Abstract
Despite its widespread use, complication of incentive spirometry has been rarely reported. We report a case of pharyngeal reperforation following incentive spirometry. A 75-year-old female, had a history of long-term steroid use, entered the intensive care unit for maintenance of mechanical ventilation following surgical repair of a pharyngeal perforation. After ventilator weaning, incentive spirometry was implemented on postoperative day 4. Immediately after incentive spirometry use, patient’s neck began to swell, and subcutaneous emphysema was palpated. Pharyngeal reperforation was suspected on neck computed tomography, and emergency surgery was performed. Surgery revealed a 3-cm long rupture from the hypopharynx to the esophagus. The causes were thought to be delayed wound healing due to long-term steroid use and a sudden increase in pharyngeal pressure due to incentive spirometry. In conclusion, particular attention should be paid when using incentive spirometry after head and neck surgery in patients with a history of long-term steroid use.
Incentive spirometry reduces pleural pressure and induces lung expansion by making the patient’s breath long, slow, and deep [1]. Consequently, this method effectively minimizes lung atelectasis, strengthens inspiratory muscles, and optimizes gas exchange [1–3]. Due to the advantages outlined above, incentive spirometry is widely used in many medical centers to reduce postoperative pulmonary complications in high-risk patients or for respiratory rehabilitation in the patients with respiratory muscle weakness [1,2]. The limitations of incentive spirometry include ineffectiveness due to inappropriate use, hyperinflation, respiratory alkalosis, fatigue, pain, and hypoxia due to interruption of oxygen supply during the procedure [1,3]. The following contraindications of incentive spirometry are known: (1) the patients in an environment where proper device use cannot be supervised; (2) uncooperative patients; (3) the patients who cannot understand how to properly use the device; (4) the patients who cannot breathe deeply in an effective way; (5) the patients with severe chronic obstructive pulmonary disease or acute asthma who are at risk of developing hyperinflation due to the limited full expiration when using the device [1,3]. However, according to our literature review, severe complications from incentive spirometry have rarely been reported; likewise, no reports are available about the precautions when applying incentive spirometry. In the present study, we report a case of pharyngeal reperforation following incentive spirometry in a patient who underwent head and neck surgery.
CASE REPORT
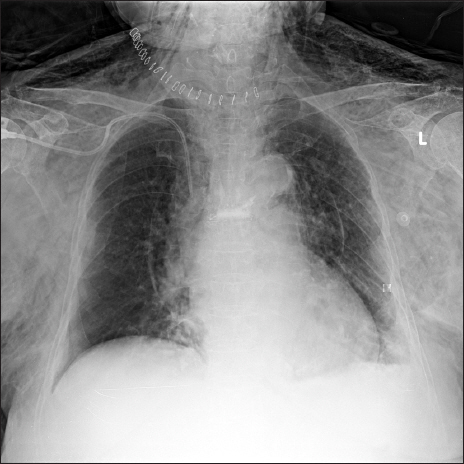
A 75-year-old female patient was admitted to the surgical intensive care unit after the surgical repair of hypopharyngeal perforation caused by a fish bone; the operation was performed under general anesthesia. The patient had hypertension, spinal stenosis, and secondary adrenal insufficiency due to long-term steroid use (prednisolone 25 mg/day for 3 years). Due to postoperative airway edema, mechanical ventilation was maintained with an endotracheal tube for four days. No abnormal signs were observed on neck computed tomography (CT) images (Figs. 1A and 1B) and during the cuff leak test. Therefore, ventilator weaning and extubation were performed on postoperative day four. The patient’s vital signs remained stable with oxygen supplied at the rate of 5 L/min using a nasal cannula. 30 minutes after extubation, volume-oriented incentive spirometry (SPIRO-BALL®, Leventon, Spain) was administered to promote lung expansion. The intensive care unit nurse monitored the patient’s appropriate use of incentive spirometry. The patient was instructed to perform slow, sustained inspiration for 5 seconds using the incentive spirometry device in the sitting position; the target inhalation volume was set to 1.7 L and the patient was instructed to use the incentive spirometry device 10 times per hour. Immediately after the application of incentive spirometry, the patient complained of neck pain and tachycardia. Subsequently, the patient’s neck swelled, and her consciousness level dropped to the drowsy state. Palpation was performed and a crackling sensation from the neck and the anterior chest wall to both upper arms was confirmed. At the time, the patient’s vital signs were as follows: heart rate, 130–175 beats/min; systolic blood pressure, 149–183 mmHg; diastolic blood pressure, 95–118 mmHg; respiratory rate, 28–30 breaths/min; and oxygen saturation (SpO2), 92–94%. The arterial blood gas analysis results are shown in Table 1. A portable chest X-ray showed extensive subcutaneous emphysema from the neck to the chest (Fig. 2). Promptly, incentive spirometry was discontinued, and intubation and mechanical ventilation were performed (3 hours and 30 minutes elapsed after extubation). Neck CT that was performed for an accurate diagnosis showed air in the retropharyngeal area and pneumomediastinum (Figs. 1C and 1D). Pharyngeal reperforation at the site of repair was strongly suspected, and emergency surgery was performed under general anesthesia. Surgery revealed a 3-cm long rupture from the hypopharynx to the esophagus. The ruptured lesion was located at the site of the first surgical repair; however, the lesion, which previously was only 1 cm, increased to 3 cm. Surgical repair and tracheostomy were performed. Five days afterwards, due to the slow healing of the wound, a rotation flap using a sternocleidomastoid muscle was performed on the lesion under general anesthesia. On day nine after the last operation, pseudomonas pneumonia developed, and the patient maintained long-term ventilator care. The patient was treated with the combination of meropenem and levofloxacin, and the symptoms of pneumonia started to improve on day 24 after the last operation. On performing neck CT and upper gastrointestinal endoscopy to confirm the absence of abnormal findings of the surgical site, ventilator weaning was performed on day 28 after the last operation. The patient was transferred from the intensive care unit to the general ward on day 31 after the last operation. Since the pneumonia was not completely cured, and the symptoms were wax and wane, the patient maintained antibiotic therapy and received respiratory rehabilitation using a cough assist machine in the general ward. However, the patient died of acute renal failure associated with septic condition 38 days after her discharge from the intensive care unit. The Pusan National University Hospital Institutional Review Board (IRB) designated this project as exempt (IRB no. H-1712-014-062).
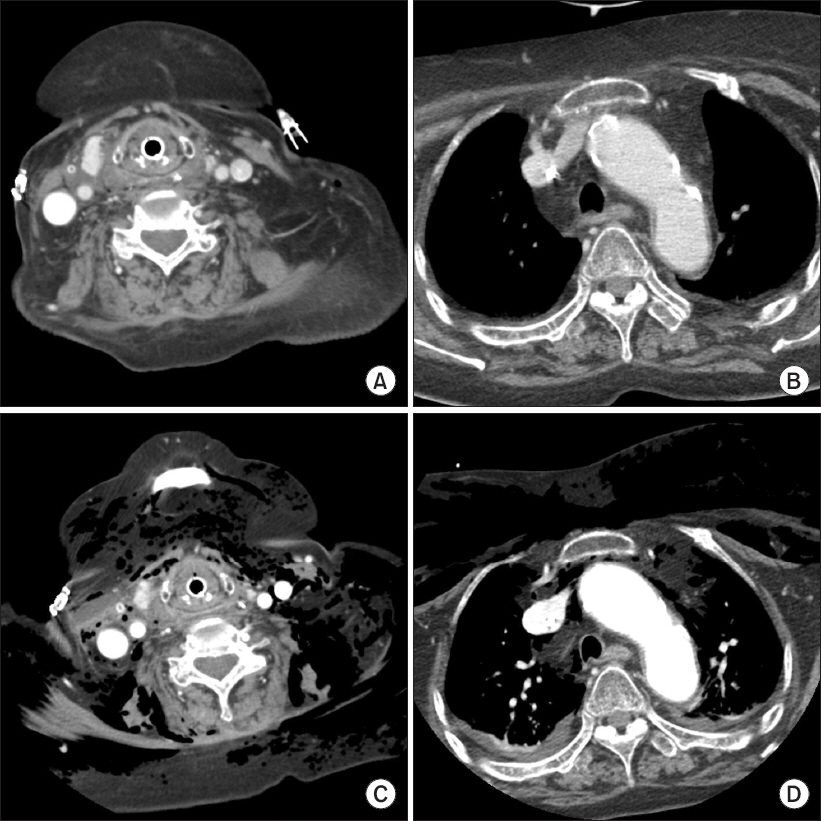
Neck computed tomography (CT) before and after incentive spirometry. Before ventilator weaning, neck CT was performed (A, B) to see the postoperative course and there were no abnormal findings. (C, D) are neck CT findings when extensive subcutaneous emphysema occurred after using incentive spirometry. Air around retropharyngeal space, pneumomediastinum, and extensive subcutaneous emphysema were observed.
DISCUSSION
The use of incentive spirometry is considered primarily to reduce postoperative pulmonary complications, for the patients who are high-risk or who are scheduled for high-risk surgery, the patients who are predicted to have difficulties with postoperative pain control, or the patients undergoing thoracoabdominal surgery [1,3]. In addition, incentive spirometry is employed for respiratory muscle training in patients with respiratory muscle weakness due to neuromuscular disease or spinal cord injury [1,2].
Both major head and neck surgery and long-term mechanical ventilation are risk factors for pulmonary complications, and previous studies have been reported the cases when incentive spirometry was applied to patients with such conditions. The incidence of postoperative pulmonary atelectasis in the patients undergoing head and neck surgery is known to be as high as 36–70% [4,5]. Tan [6] applied incentive spirometry to the patients who underwent laryngectomy or major head and neck surgery requiring tracheostomy. In that study, the postoperative lung function of the incentive spirometry group was well preserved as compared to that of the control group. Prolonged mechanical ventilation is another pulmonary complication-inducing factor. Mechanical ventilation causes atrophy of diaphragm myofibrils by disuse, and the diaphragm thickness is known to decline by 10.9% per mechanical ventilation day [7]. Therefore, to restore the patient’s pulmonary function after ventilator weaning, diaphragm muscle training should be performed. Sawant and Shinde [2] applied incentive spirometry for diaphragm muscle training after mechanical ventilator weaning. They reported an improvement on the pulmonary function test and an improvement of the American thoracic society dyspnea scale score in the patients using incentive spirometry.
Despite the widespread use, the efficacy of applying incentive spirometry after surgery remains controversial [8]. In this regard, Cassidy et al. [9] have suggested the I COUGH program, a multidisciplinary patient care program, as a novel method to reduce postoperative pulmonary complications. The I COUGH program consists of the following components: incentive spirometry, coughing and deep breathing, oral care, understanding (patient and family education), getting out of bed frequently (at least 3 times daily), and head-of-bed elevation. The authors reported that, owing to the application of the I COUGH program, the incidence of pneumonia and unplanned re-intubation was reduced after surgery. Therefore, rather than being applied alone, incentive spirometry should be used as part of the multidisciplinary program. In our case, the patient also received the multidisciplinary patient care program after ventilator weaning. The patient had application of incentive spirometer, oral care, understanding, and head-of-bed elevation; yet, due to general weakness resulting from the long-term bed ridden state, the patient was unable to get out of bed frequently.
Pharyngeal perforation is usually caused by foreign body ingestion or iatrogenic causes such as endoscopic procedures; therefore, cases of spontaneous pharyngeal perforation by other miscellaneous causes are extremely rare [10–12]. Pharyngeal perforation occurs when the internal pressure of the pharynx suddenly and excessively increases, thereby leading to a rupture of the weak part of the pharyngeal wall by the pressure [10,11]. In the present report, the pharyngeal perforation is also thought to be caused by the above mentioned two factors, i.e. weakening of the pharyngeal wall and the subsequent development of the pharyngeal pressure beyond the durability of the pharyngeal wall. As to the first factor, the patient’s medication history, which included the long-term use of a steroid, may have contributed to the delaying wound healing and weakening of the pharyngeal wall. Specifically, corticosteroids are known to affect all three phases of wound healing—inflammatory, proliferative, and remodeling—which results in a reduced wound tensile strength and slow wound healing [13]. Previous research has reported that the use of chronic corticosteroids for a period exceeding 30 days before surgery causes a 2-3-fold increase in the risk of wound dehiscence, as well as a 2-5-fold higher rate of wound complications [13,14]. In a similar case, Gorosh et al. [15] reported a spontaneous tracheal wall rupture after cough in a giant cell arteritis patient who had used steroids for 8 years. Another cause of the tracheal rupture, as suggested by the authors, was connective tissue fragility due to the long-term use of steroids. Second, an increase of pharyngeal pressure was thought to be due to the use of incentive spirometry. Incentive spirometry has been devised to mimic natural sigh or yawning [1]. Therefore, when incentive spirometry is applied, patients are instructed to inhale as deep as possible, hold breath for several seconds, and exhale slowly. During this process, the increase in airway pressure can occur in both deep inspiration and breath holding. Definitely, the upper airway pressure caused by incentive spirometry is not sufficiently excessive to cause perforation in normal pharyngeal tissue. However, in the present case, the patient had already undergone pharyngeal repair surgery and had a history of long-term steroid use. Due to these factors, the postoperative wound healing was significantly delayed and the fragility of the patient’s connective tissue was also aggravated. Unlike in the normal pharyngeal tissue, durability and pressure threshold that can be sustained without damage in the pharyngeal tissue of this patient may have been significantly reduced. Similarly, Kim et al. [10] reported that, in the context of acute pharyngitis, even shouting can induce spontaneous pharyngeal perforation.
Before extubation, no abnormal findings in the postoperative neck CT before the ventilator weaning were observed. Also, the patient’s symptoms didn’t develop after extubation, but after applying incentive spirometry. Considering these circumstances, a relatively large, 3-cm long, pharyngeal rupture should have occurred after, rather than before the application of incentive spirometry. Another possibility to consider is that there was a small, radiologically undetected, and asymptomatic defect that exacerbated into the 3-cm long rupture by the use of incentive spirometry. Accordingly, incentive spirometry appears to have contributed to the development or exacerbation of the patient’s pharyngeal reperforation.
Our results suggest that physicians should pay particular attention to the application of incentive spirometry in the patients undergoing head and neck surgery and receiving long-term corticosteroid therapy. In addition, special attention should be paid to the patients with these medical conditions to avoid vomiting, shouting, coughing, or sneezing, which may increase internal pressure of the pharynx after surgery. Although the incidence of unexpected pharyngeal re-perforation is very low, additional evaluation for an accurate diagnosis should be urgently performed if symptoms develop after head and neck surgery.
ACKNOWLEDGMENTS
This work was supported by a 2-Year Research Grant of Pusan National University.