Liver disease affects many organ systems including the hemostatic system [
1,
2]. Moreover, impaired hepatic synthesis of thrombopoietin, splenic sequestration, and rapid turnover of thrombocytes often cause thrombocytopenia in end-stage liver disease (ESLD) patients. Because of this, in the past, patients with cirrhosis have been thought of as in an ŌĆ£auto-anticoagulated state.ŌĆØ However, because there is a relative deficiency of both pro-coagulant and anti-coagulant factors in ESLD patients, this classical interpretation has been replaced by the concept of ŌĆ£rebalanced hemostasis.ŌĆØ This concept was firstly mentioned by Lisman and Porte [
3] in 2010. According to this concept, the hemostatic alterations in chronic liver disease result in a new balance. However, this balance is much more fragile and can shift easily toward either bleeding or thrombosis [
3,
4]. Assessment of the risk of bleeding in ESLD patient is not easy by conventional coagulation tests due to their inherent limitations. Viscoelastic tests such as thromboelastography (TEG) or rotational thromboelastometry (ROTEM) provide information on the overall coagulation system and therefore, may be better tools to gauge the risk of bleeding than conventional coagulation tests [
4]. We report a case of a patient who had severe thrombocytopenia (19 ├Ś 10
9/L) due to ESLD but underwent major surgery without any intraoperative and postoperative transfusions.
CASE REPORT
A 44-year-old man (body weight 73.9 kg, height 174.6 cm, type O blood) with combined hepatocellular carcinoma (HCC) and cholangiocarcinoma with liver cirrhosis was admitted for laparoscopic mass excision. Computed tomography showed splenomegaly and an extrahepatic- mass, which was about 2.8 cm in diameter and located in the perihepatic space, adjacent to liver segment 6 (
Fig. 1). The patientŌĆÖs Child-Pugh class was B with a score of 7. He had been treated with a transarterial chemoembolization procedure three times and had underwent a wedge resection and lobectomy for lung metastasis of HCC. The preoperative laboratory value showed a hemoglobin level of 11.8 g/dl, hematocrit of 34%, platelet count of 19 ├Ś 10
9/L, prothrombin time (PT) of 19.3 seconds with an international normalized ratio (INR) of 1.60, and activated partial thromboplastin time (aPTT) of 44.1 seconds. Other laboratory tests including pulmonary function test showed normal findings. After discussing with the surgeon, it was decided that the patient would not receive prophylactic platelet transfusion despite the very low platelet count and to do a transfusion during surgery if necessary.
Fig.┬Ā1
Computed tomography (CT) of the the extrahepatic mass. CT shows a slight increase in the size (2.8 cm) of the presumed seeding nodule in the peri-hepatic space, adjacent to liver segment 6 with splenomegaly.

Electrocardiography, pulse oximetry and noninvasive blood pressure were applied before induction, and the initial vital signs showed a blood pressure 120/70 mmHg, a heart rate of 90 beats/min, and a pulse oximeter saturation of 100%. Anesthesia was induced with intravenous thiopental sodium 350 mg and vecuronium 10 mg and maintained with sevoflurane. Airway maintenance was done with a laryngeal mask airway (Protector, Teleflex Medical, Japan). Right radial artery was cannulated, and there were no hemodynamic instabilities during the induction. After the induction, tests for the platelet count, the plasma von Willebrand factor (vWF) level, and ŌĆ£real timeŌĆØ coagulation status using the rotational thromboelastometry (ROTEM Analyzer, Tem International GmbH, Germany) were done. The platelet count was 24 ├Ś 109/L and, the plasma vWF antigen level was 179% (reference range: 52-155% for blood group O). The ROTEM (NATEM) showed a clotting time (CT) of 683 seconds (reference range: 300-1,000 seconds), a clot formation time (CFT) of 716 seconds (reference range: 150-700 seconds), an alpha angle of 31┬░ (reference range: 30-70┬░), a maximum clot firmness (MCF) of 26 mm (reference range: 40-65 mm), and a LI60 of 91% (reference range: 76-96%).
After placing the surgical retractor, we changed the anesthetic gas from sevoflurane to isoflurane. Vital signs and other monitoring parameters including end-tidal carbon dioxide, lactate, and electrolytes were within normal range. The procedure was converted from laparoscopic to open due to adhesions around the right kidney, but the patient did not show any sign of bleeding tendency. The total anesthesia time was 2 hours and 21 minutes, and only 700 ml of Plasma Solution A
┬« (CJ HealthCare Corp., Korea) were intravenously administered. The estimated blood loss was 270 ml, and total urine output was 50 ml. We assessed the patientŌĆÖs coagulation status using complete blood count and ROTEM (NATEM) tests at the end of the surgery. The results were a hemoglobin level of 12.1 g/dl, a hematocrit level of 36%, and a platelet count of 24 ├Ś 10
9/L. The ROTEM values were similar to the initial values, with a CT of 698 seconds, a CFT of 439 seconds, an alpha angle of 38┬░, a MCF of 32 mm, and a LI60 of 93% (
Table 1).
Table┬Ā1
Flow Chart of the Coagulation Tests
|
ŌĆāTime of sampling |
Platelet (/L) |
CT (s) |
CFT (s) |
╬▒(┬░) |
MCF (mm) |
LI60 (%) |
vWF (%) |
|
Preoperative 1 day |
19 ├Ś 109
|
- |
- |
- |
- |
- |
- |
|
After induction (8:25 a.m.) |
24 ├Ś 109
|
683 |
716 |
31 |
26 |
91 |
179 |
|
End of operation (10:20 a.m.) |
24 ├Ś 109
|
698 |
439 |
38 |
32 |
93 |
- |
|
Postoperative 1 day (8:00 a.m.) |
24 ├Ś 109
|
- |
- |
- |
- |
- |
- |
The patient was transferred to the post-anesthesia care unit after surgery and was discharged on the 6th postoperative day (POD) without any complications and transfusions. The antithrombin III value was 68% (reference range: 83-123%) on the 80th POD.
DISCUSSION
Chronic liver disease results in complex alterations of all phases of hemostasis, and the net effect is considered to be a rebalanced hemostasis [
3,
4]. Deficiencies in pro-coagulant proteins are balanced by deficiencies in anti-coagulant proteins such as protein C and S and anti-thrombin [
3]. Thrombocytopenia, a typical feature of chronic liver disease, is balanced by increased platelet function with high levels of vWF and low levels of ADAMTS 13 which cleaves vWF [
3,
4]. The fibrinolytic system may also be rebalanced by the concomitant decrease of antifibrinolytics (antiplasmin and thrombin activatable fibrinolysis inhibitor) and plasminogen [
4,
5]. In this case, the patient showed a rebalanced hemostasis: The decrease in pro-coagulant levels (increased PT) was balanced by a decrease in antithrombin III levels, and the low platelet count was balanced by increased levels of vWF and no hyperfibrinolysis on the ROTEM.
Conventional coagulation tests do not fully reflect the derangement in hemostasis in ESLD patients. For example, PT and aPTT tests are not sensitive to the deficiency of anti-coagulants. The PT test only assesses the function of a discrete number of pro-coagulant proteins (factors VII, X, V, and II and fibrinogen) and therefore cannot reflect the true hemostatic status of a patient because it does not consider the function of the protein C and S pathway, antithrombin, and tissue factor pathway inhibitor [
4,
5]. Thus, moving away from the traditional laboratory measures of coagulation such as PT, aPTT and platelet counts to newer functional viscoelastic measures of coagulation such as TEG or ROTEM may be better for assessing coagulopathy in patients with chronic liver disease who undergo surgery [
4-
6]. TEG or ROTEM helps identify different coagulation defects including hyperfibrinolysis, hypofibrinogenemia, thrombocytopenia and hypercoagulopathy. These tests are point-of-care coagulation monitoring systems that rapidly evaluate the viscoelasticity of the whole blood, enabling the entire clotting process, from clot initiation and formation to clot stability, to be assessed [
7-
9].
Prophylactic platelet transfusion for patients having major elective non-neuraxial surgery is recommended if the platelet counts are less than 50 ├Ś 10
9/L. For neurosurgery, platelet transfusion is recommended if the platelet counts are less than 10 ├Ś 10
10/L [
10]. However, this recommendation should be applied with caution in ESLD because of the rebalanced hemostasis. Actually, patients undergoing liver transplantation in our hospital receive single donor platelets (SDP) only when the platelet count is below 30 ├Ś 10
9/L. In this case, we did not perform any prophylactic transfusion even though the laboratory results revealed severe coagulopathy with a PT of 19.3 seconds, an INR of 1.60, an aPTT of 44.1 seconds, and a platelet count of 19 ├Ś 10
9/L. The patient had previously undergone lung surgery twice, and had showed similar preoperative conventional coagulation tests such as the PT, aPTT, and platelet counts. In the previous two major surgeries, two and five units of SDP were administered before or during surgery, respectively, and the platelet counts increased to only about 10 ├Ś 10
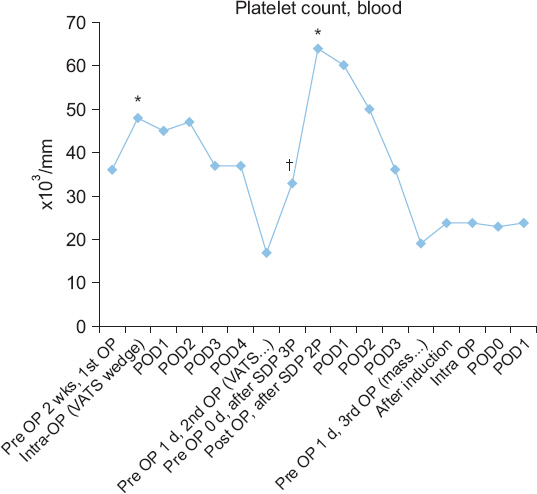
9/L with 1 unit of SDP, and the final platelet counts were below the normal range in both cases (
Fig. 2). However, the estimated blood loss was only 100 and 200 ml during the first and second surgeries, respectively, and there was no postoperative bleeding complication in both surgeries. From this, we had expected that the patient might have a rebalanced hemostasis. We closely observed the bleeding tendency in the surgical field and performed ROTEM tests to determine the patientŌĆÖs actual coagulation status instead of relying on the conventional coagulation tests. The ROTEM result during surgery revealed a near normal coagulation status, and reflected the actual bleeding tendency in the surgical field better than the conventional coagulation tests.
Fig.┬Ā2
Variation in the platelet counts after transfusion of single donor platelets (SDP). OP: operation, POD: postoperative day. *Platelet counts after 2 units of SDP transfusion. ŌĆĀPlatelet counts after 3 units of SDP transfusion.
In conclusion, if an ESLD patient is suspected of having a rebalanced hemostasis, ROTEM can be a more useful coagulation monitoring tool than the conventional coagulation tests. Moreover, it may be helpful to use ROTEM when making decisions about prophylactic transfusions.










