Intraoperative paravalvular leakage after sutureless aortic valve replacement corrected with secondary balloon dilatation -A case report-
Article information
Abstract
Sutureless aortic valve replacement was performed in a 72-year-old female patient with severe aortic stenosis who had undergone coronary revascularization and pacemaker implantation. After valve excision, decalcification was deliberately incompletely performed at the commissure of the left- and non-coronary cusp to obtain a regular and circular annular margin. After implantation of the stented valve, no paravalvular leakage was noted on water irrigation testing. Upon weaning from cardiopulmonary bypass, a moderate degree of paravalvular leakage was observed by transesophageal echocardiography at the junction of the left- and non-coronary cusp. Instead of removing the valve and performing more complete decalcification to implant a larger valve, secondary balloon dilatation and warm sterile water irrigation were performed to allow further expansion and fixing of the metal alloy stent around the aortic wall to minimize the duration of aortic cross-clamp. No paravalvular leakage was observed thereafter and the patient was discharged without any complications.
INTRODUCTION
In patients with severe aortic stenosis, sutureless aortic valve replacement (SU-AVR) has emerged as a valuable technique that allows a reduction in cardiopulmonary bypass (CPB) time and global risk profile related to surgery in high-risk patients [1]. SU-AVR has been shown to provide an excellent hemodynamic profile with comparable rates of complications, including paravalvular leakage, to that of conventional aortic valve replacement while allowing a significant reduction in the aortic cross-clamp time [2]. As the stenotic calcified valve is excised before SU-AVR, intraoperative paravalvular leakage is rare and only cases of delayed postoperative stent distortion have been reported to date [3]. This is the first report of a case of intraoperative paravalvular leakage, possibly due to deliberate incomplete decalcification to obtain a regular and annular margin, that was readily detected by intraoperative transesophageal echocardiography examination upon weaning from CPB. Leakage was successfully managed by secondary balloon dilatation and warm water irrigation to allow further expansion of the stented prosthetic valve without new valve replacement.
CASE REPORT
A 72-year-old woman (height 148 cm, weight 53.9 kg) was scheduled for aortic valve replacement due to symptomatic severe aortic stenosis. She underwent right-sided mastectomy in 2000, off-pump coronary artery bypass surgery in 2006, and pacemaker insertion (VVI mode) in 2012. Preoperative echocardiography revealed severe aortic stenosis (aortic valve area: 0.75 cm2, peak systolic pressure gradient [PSPG]/mean systolic pressure gradient [MSPG]: 94/42 mmHg) due to calcific degeneration, grade 2 tricuspid regurgitation (annular diameter of 33 mm), right ventricular systolic pressure of 48 mmHg, and preserved left ventricular ejection fraction of 63%. Preoperative computed tomography revealed right-sided pleural effusion, apical fibrosis of the right lung, obstructed left brachiocephalic vein, patent previous coronary bypass grafts, and an aortic annular diameter of 22.4 mm.
Upon redo median sternotomy, the right ventricular free wall was perforated and repaired. CPB was performed at a body temperature of 32–34°C with α-stat pH management. Cardioplegic arrest was achieved with cold blood cardioplegia. The calcified stenotic native valve was excised and incomplete decalcification was deliberately performed at the commissure of the left- and non-coronary cusp to ensure a regular and circular annular profile for valve implantation. The surgeon re-evaluated the aortic annulus size with valvular sizers and confirmed the appropriate SU-AVR valve size. The aortic valve was replaced with a medium sized sutureless aortic valve (Perceval S®, Sorin group, Saluggia, Italy) covering annular diameters of 21–23 mm. The prosthesis was checked visually after crimping, deployment, and balloon dilatation according to the manufacturer’s protocol; ballooning was performed with pressure of 4 atm for 30 seconds. Before closing the aortotomy incision, careful inspection for paravalvular leakage was performed by the surgeon using warm saline irrigation and no leakage was noted.
Upon weaning from cardiopulmonary bypass, paravalvular leakage of moderate degree was noted by transesophageal echocardiography examination at the junction of the left-and non-coronary cusp side with a visible gap of approximately 3 mm (Fig. 1 and Video 1).
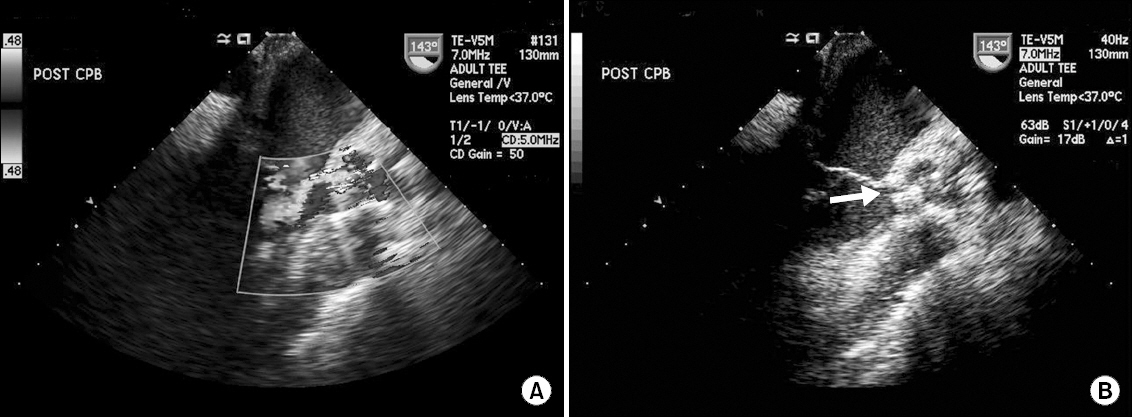
Upon weaning from cardiopulmonary bypass, paravalvular leakage of moderate degree was noted by transesophageal echocardiography examination at the junction of the left-and non-coronary cusp side with a visible gap of approximately 3 mm.
The ascending aorta was opened again for correction of the paravalvular leakage. The gap at the junction of the left-and non-coronary cusp was not evident and there was no distortion of the stented valve. Instead of removing the seated valve, secondary balloon dilatation and warm saline irrigation was performed. Secondary balloon dilatation was performed by addition of an extra 2 ml of normal saline after achieving a pressure of 4 atm. Second weaning from CPB was uneventful with no paravalvular leakage detected by transesophageal echocardiography (Video 2). Total duration of aortic cross-clamp was 56 min.
The patient stayed in the intensive care unit for 10 days because of pneumonia and right ventricular dysfunction, which were managed with antibiotics and dobutamine. She was discharged without any complications after a postoperative hospital stay of 21 days. Postoperative transthoracic echocardiography performed 1 day prior to discharge revealed a well-functioning stented bioprosthetic aortic valve (PSPG/MSPG: 30/13 mmHg), grade 2 tricuspid regurgitation, right ventricular systolic pressure of 43 mmHg, and a left ventricular ejection fraction of 55%.
DISCUSSION
As we face an increasingly aging society, the prevalence of aortic stenosis is increasing and greater numbers of elderly patients with various co-morbid diseases present for cardiac surgery. In addition to patient-related risk factors, prolonged durations of CPB and aortic cross clamp are well-known strong independent risk factors for postoperative mortality and morbidity [4,5].
SU-AVR using a stented bioprosthetic valve offers significant reductions in both CPB and aortic cross-clamp times, which may potentially lead to improved outcome [6]. In contrast to transcatheter aortic valve replacement (TAVI), SU-AVR is performed after valve excision and results in optimal hemodynamic performance in terms of the effective valve orifice area and similar early and long-term complication rates compared to conventional aortic valve replacement requiring annular sutures [2]. A case of leakage caused by postoperative stent distortion has been reported, and stent fatigue and creep have been proposed as potential concerns [3]. Nonetheless, the reported incidence of early and late postoperative paravalvular leakage ranges from 2% to 4%, which is far lower than that of TAVI and comparable to that of conventional aortic valve replacement [7,8].
After careful sizing and positioning of the stented valve, balloon dilatation is performed to expand the superelastic metal alloy stent, followed by warm water irrigation to expand and fix the frame within the appropriate annular position [9]. As the native stenotic valve is excised and the stented valve is implanted under direct visualization, intraoperative paravalvular regurgitation can usually be detected by the surgeon immediately after valve implantation. Accordingly, no case of intraoperative detection of paravalvular leakage after SU-AVR has been reported.
In the current case report, no visible gap or paravalvular leakage was observed by the surgeon upon visual inspection and water irrigation testing before closing the ascending aorta. However, a moderate degree of paravalvular leakage could be observed at the incompletely decalcified annular site, which was deliberately generated to ensure a regular and circular annular profile. The actual shape of the aortic annulus is somewhat oval in most patients whereas the annular profile of the prosthetic valves is round. To prevent late stent distortion or paravalvular leakage in SU-AVR, more emphasis is given to obtaining a regular and circular annular profile and complete decalcification is not necessary in this context [3].
Caution should be exercised in cases of deliberate incomplete annular decalcification, such as described here, because the water irrigation test does not ensure lack of paravalvular leakage. Indeed, the pressure generated by the small water column after valve implantation would be negligible, whereas the actual diastolic pressure after weaning from CPB could range from 50 mmHg to 80 mmHg. Therefore, a gap between the stented valve and the calcified annulus may not be evident on visual inspection or water irrigation test. Despite use of a stented valve SU-AVR is known to be associated with minimal risk of paravalvular leakage, in contrast to TAVI [1]. However, this case clearly highlights the importance of a careful comprehensive intraoperative transesophageal echocardiography examination and the attending anesthesiologist should be aware of the possibility of unexpected paravalvular leakage. Transesophageal echocardiography is not only performed to monitor the hemodynamic state of the patient, but also to guide the operation and estimate the outcome of surgery [10]. As it provides a chance to correct any failure in advance, it affects the prognosis of patients. The ability of anesthesiologists to control transesophageal echocardiography is therefore essential.
Sutureless valves have a restricted range of sizes: size S, to be implanted in annular sizes from 19 to 21 mm, size M for 21–23 mm, and size L for 23–25 mm [2]. Removal of the valve and further decalcification to introduce a bigger valve might have been an option to address the paravalvular leakage. Also, in the case of evident stent distortion a smaller sized valve may be chosen while considering the switch to a conventional aortic valve replacement. As demonstrated by the current case, secondary ballooning may be useful to correct the paravalvular leakage when no definite stent distortion is noted and a proper sized sutureless valve was implanted.
Although SU-AVR has been shown to be associated with a significant reduction in the length of intensive care unit and hospital stays [6], and consequently a reduction in medical costs, this approach may not be applicable in countries with different insurance policies when considering the high cost of the stented valve. More importantly, the purpose of SU-AVR is to reduce the duration of aortic cross-clamp and consequently the global risk profile related to surgery. Overall, in cases of paravalvular leakage despite the use of a correct sized valve at a regular margin of annulus obtained by incomplete decalcification, secondary ballooning and warm water irrigation may be attempted to induce further expansion of the stented valve to prevent paravalvular leakage while minimizing the aortic cross clamp time and medical costs.