The oropharyngeal bleeding after esophageal stethoscope insertion -A case report-
Article information
Abstract
The daily insertion of endotracheal tubes, laryngeal mask airways, oral/nasal airways, gastric tubes, transesophageal echocardiogram probes, esophageal dilators and emergency airways all involve the risk of airway structure damage. In the closed claims analysis of the American Society of Anesthesiologists, 6% of all claims concerned airway injury. Among the airway injury claims, the most common cause was difficult intubation. Among many other causes, esophageal stethoscope is a relatively noninvasive monitor that provides extremely useful information. Relatively not many side effects that hardly is ratable. Some of that was from tracheal insertion, bronchial insertion resulting in hypoxia, hoarseness due to post cricoids inflammation, misguided surgical dissection of esophagus. Also oropharyngeal bleeding and subsequent anemia probably are possible and rarely pharyngeal/esophageal perforations are also possible because of this device. Careful and gentle procedure is necessary when inserting esophageal stethoscope and observations for injury and bleeding are needed after insertion.
INTRODUCTION
One of the major causes of airway injury during anesthesia is tracheal intubation. Other causes include the use of a laryngeal mask airway, an artificial airway, an L-tube, transesophageal echocardiogram, esophageal dilator and any other emergency airway managements [1]. According to a closed claims analysis by the American Society of Anesthesiologists (ASA), difficult tracheal intubation is the most common cause of airway injury, accounting for 39 percent of medical malpractice lawsuits regarding airway injury. Difficult tracheal intubation was a more common cause of injuries to the pharynx or esophagus, responsible for 51 percent and 62 percent of cases, respectively. Types of pharyngeal trauma include pharyngeal perforation (37%), laceration or blunt trauma (31%), and local infection (12%). Causes of perforation other than difficult tracheal intubation include the use of an L-tube, an aspiration catheter and jet ventilation. Other reported causes are esophageal dilator, using an esophageal stethoscope, esophageal intubation and the use of laryngoscope during surgery [2]. An esophageal stethoscope has often been used as an effective alternative to a precordial stethoscope in surgery on the thorax or for craniocervical surgery, or in surgeries in which patients are in a prone position, when it is difficult to use precordial stethoscope [3]. Moreover, the esophageal stethoscope is more suitable than a precordial stethoscope for in collecting information about myocardial contractility, systemic vascular resistance and the pulmonary artery pressure through the digital analysis of heart sounds, as it generates less noise than its counterpart [4]. Furthermore, the esophageal stethoscope is useful in that it can be used to measure the core temperature of the distal esophagus with an esophageal temperature probe built into the device [5]. Side effects of using an esophageal stethoscope that have been reported include tracheal intubation [3,6], endobronchial intubation and hypoxia that follows [7], pressuring the recurrent laryngeal nerve on the posterior part of cricoid cartilage leading to vocal cord paralysis [8] and cases in which the esophagus with a stethoscope is mistaken for the internal jugular vein with a catheter and thus cut [3]. We report our experience with a suspected case of an oropharyngeal laceration stemming from the use of an esophageal stethoscope.
CASE REPORT
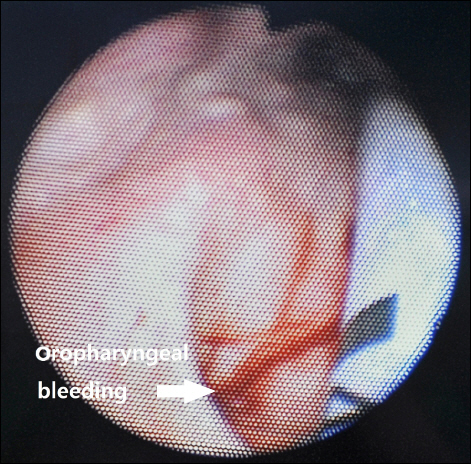
The patient was a 64‐year‐old male with a height of 172 cm and a weight of 60 kg, without abnormality in his medical history or in his family history. He fell from a tree 2 m high and was given conservative treatments from other hospital. The patient was transferred to our hospital three months after the injury, after he was recommended to visit a tertiary hospital for surgery of left bitrochanteric fracture and burst fracture on L1. He showed normal vitals after hospitalization and before the surgery, with a systolic blood pressure of 100 to 130 mmHg, a diastolic blood pressure of 60 to 80 mmHg, a heart rate of 70 to 90 beats/min, and body temperature of 36.5–37°C. At the time of hospitalization, his bilirubin was measured at 3.5 mg/dl, serum albumin at 27 g/L, PT INR (prothrombin time international normalized ratio) at 1.04 and preoperative hemoglobin level was 10.7 g/dl. The patient was found to have liver cirrhosis classified as Child‐Pugh class B without findings for ascites, esophageal varices or hepatic encephalopathy. A chest X‐ray examination before the surgery found mild pulmonary edema; a pulmonary function test showed mild restrictive pulmonary disease; and a brain computed tomography showed a subdural hygroma on convex surfaces of both the frontoparietal areas and local bleeding in the right parietal lobe. The patient entered the operation room to undergo open reduction and internal fixation surgery of the left bitrochanteric fracture. An intramuscular injection of glycopyrrolate 0.2 mg was given as a premedication 1 hour prior to the initiation of the operation. Upon arrival into the operation room, the blood pressure was measured every 3 minutes with a noninvasive blood pressure device for safety, electrocardiogram (lead II, lead V5) was constantly monitored, and pulse oximetry and capnography were used to monitor the patient. The patient was in slightly drowsy mentality when he arrived in the operation room, and the early blood pressure was 118/76 mmHg, with a pulse rate of 87 beats/min and an oxygen saturation level of 97%. After 3 minutes of oxygenation before anesthesia and confirmation that the oxygen saturation level was maintained at 100%, 120 mg of propofol was injected, with 60 mg of lidocaine was injected at the same time to reduce the pain of the propofol injection. After the patient was confirmed to have lost consciousness, succinylcholine 100 mg was injected to induce muscular relaxation. Due to his poor dental health, fiberoptic bronchoscopy was used to intubate the tracheal tube (Mallinckrodt™ 7.5 mm I.D., Covidien IIc, USA). Fiberoptic bronchoscope was inserted to tracheal tube until it reached slightly before end of tube to avoid laceration due to the sharp end of bronchoscope. Thereafter, when tip of the scope advanced until it is beyond the base of the tongue, a jaw thrust provided by an assistant and then authors could see oropharynx, esophageal opening, and glottic opening easily. When passing through the vocal cord, felt no resistance. After orotracheal intubation, dental injury or bleeding and oral cavity injury or bleeding was not found. And then oropharyngeal airway (AirWay 96 mm [#4], Ace Medical, Korea) was inserted. During inserting airway, there was no resistance. Thereafter, we underwent oral suction, but there are no findings that represent the oral bleeding. Since in the preoperative examination respiratory tract problems were discovered and hypothermia is possible due to massive bleeding during surgery, we were judged continuous auscultation and temperature measurements are required. Fortunately, the patient had liver cirrhosis, but he did not have esophageal varices and bleeding. So that after anesthesia induction, an esophageal stethoscope (Esophageal Stethoscope w/Temperature Sensor, DeRoyal Industries Inc., USA) was inserted through the mouth, and although there was slight resistance at the oropharynx, spiraling the stethoscope it is not very difficult to be inserted. A surgical lubricant (SURGㆍJELLE, Bio‐Chem, Laboratories Corp., USA), was applied enough from tip to 7 cm of the stethoscope from the tip to ease the insertion. After inserting the stethoscope to 30 cm inside from the upper lip and confirming that there was no strangulation or bleeding on the oral cavity, the end of the stethoscope out of the mouth was fixed to an artificial airway. Moreover, a bispectral score (BIS) sensor (BIS™ Quatro, Covidien, Korea) and a noninvasive cardiac output monitor (NICOM® Sensors, Cheetah Medical, USA) were used to monitor the consciousness and vitals of the patient. Oxygen 2 L/min and N2O 2 L/min along with a 6% desflurane vaporization concentration were maintained to sustain anesthesia. A total of 8 mg of vecuronium was injected as an additional muscle relaxant ― 4 mg immediately after the endotracheal intubation, 2 mg immediately before the incision and 1 mg every 30 to 40 minutes afterwards. In this operation, only closed reduction the left bitrochanteric fracture was performed, with surgery of the fractures on L1 scheduled one month later. During the 3 hours and 15 minutes of anesthesia, a systolic blood pressure of 90 to 130 mmHg, a diastolic blood pressure of 55 to 85 mmHg and a pulse rate of 60 to 90 beats/min were maintained, except when the blood pressure rose to 167/103 mmHg immediately after the endotracheal intubation. BIS was maintained between 35 and 45. While the cardiac index stayed in the normal range of 2.5 to 3 L/m2 in NICOM, the stroke volume variation was higher than normal, at 16 to 17 percent, which can be attributed to the insufficient intake of food after the injury for months and dehydration due to NPO before the surgery. During the operation, 400 ml of crystalloid fluids (Plasma solution A inj., CJ Health Care, Korea) and 1,000ml of a starch plasma volume expander (6% Volulyte inj., Fresenius KABI, Korea) were injected. No further injections of fluids were given, as they could have worsened the pulmonary edema, and the dehydration state was not severe considering the 100 ml of urine output per hour. However, we recommended that the orthopedic department give fluids when the chest X-ray showed that the pulmonary edema had eased after surgery. The hemorrhage volume at the surgical site was about 800 ml. After the 2 hours and 25 minutes of the operation, 15 mg pyridostigmine was injected as an antagonist along with 0.4 mg of glycopyrrolate. Then, when the stethoscope was removed, there was red blood on the tip. Endotracheal and intraoral suction was performed, and while there was no blood found from the endotracheal suction, the dilute secretions from intraoral suction showed a small amount of blood. After 5 minutes following the suction, the patient’s spontaneous breathing was recovered, and his consciousness was back close to the state before the operation, after which extubation was performed. When extracting tracheal tube and artificial airway, there was no visible blood on there. After monitoring for 3 minutes with oxygenation, the patient was transferred to the recovery room. Although he intermittently coughed out blood in the recovery room, he was only monitored without treatment, as the amount of bleeding was not significant and was decreasing over time. After confirming that there was no intraoral bleeding, the patient was transferred to a general ward 25 minutes after he was moved from the recovery room. In a blood count test 1 hour after the operation, the hemoglobin level was found to be 9 g/dl. The patient, however, was unstill and making unclear complaints 2 hours and 30 minutes after the operation. We examined him as he began coughing out bloody sputum, finding that his blood pressure was 80/60 mmHg, and his hemoglobin level was 8.1 g/dl. Accordingly, a packed red blood cell transfusion was given. The hemoglobin level was 6.2 g/dl after 1 unit of packed red blood cells was given; therefore, an additional 3 units of packed red blood cells and 2 units of fresh frozen plasma were transfused. The blood pressure was normalized to 110/80 mmHg for a while, but it dropped again to 80/60 mmHg at 3 hours and 30 minutes after the operation. Accordingly, a dopamine infusion (16.1 μg/kg/min) was administered. Irrigation after L‐tube insertion was bloody. After 10 minutes, the blood pressure was monitored at 70/40 mmHg, and we increased the dopamine infusion rate to 24.1 μg/kg/min as a result. A laryngoscopic examination conducted 4 hours and 30 minutes after the operation showed a linear laceration (1 cm wide and 1 to 2 mm deep) (Fig. 1), on which hemostasis was achieved with epinephrine/lidocaine gauze. Then, a norepinephrine infusion (0.16 μg/kg/min) was used as the blood pressure dropped to 80/50 mmHg, which was normalized to 110/70 mmHg after the treatment. The dopamine and norepinephrine infusion rates were gradually adjusted lower until the norepinephrine and dopamine infusions were stopped 31 hours and 47 hours after the surgery, respectively. The amounts of blood in the sputum and hematemesis on the first day after the surgery were less than 1 L. No additional examinations such as endoscopy were performed because there was no bleeding around the L-tube, and the patient showed stable vitals. The chest X-ray on the third day after the operation showed slight exacerbation of the pulmonary edema, while there were no other symptoms such as shortness of breath or indication of aspiration pneumonia. A chest X-ray which was taken 12 days after surgery showed that the pulmonary edema had almost disappeared. The patient was discharged 19 days after the operation as the surgical site had healed without problems. Thereafter about 3 months from discharge, the patient took an esophagogastroduodenoscopy and there was no problem found but atrophic gastritis, that we considered the possibility of esophageal varix bleeding to be very low.
DISCUSSION
In the present case, oropharyngeal bleeding could be occurred several different causes. However, due to patient’s dental problem, we use fiberoptic bronchoscopy to intubate the tracheal tube instead of using laryngoscope that is considered one of common cause of airway injury. After orotracheal intubation, dental injury or bleeding and oral cavity injury or bleeding was not found and we could not even see the bleeding by suctioning oral cavity. Thereafter, oropharyngeal airway and esophageal stethoscope was inserted. When removing these devices, tracheal tube and oropharyngeal airway were no visible blood on there. Esophageal stethoscope was visible blood on the tip. In addition, resistance is detected only in the process of inserting the esophageal stethoscope; other procedures have been implemented without resistance.
Pharyngeal perforation due to the use of an esophageal stethoscope has been reported, although rarely [2,9], but pharyngeal laceration followed by bleeding is an extremely rare case. In this case, intubation and extubation was done with ease and without any perceived problem. Thereafter the mistake in this case was that an injury to the pharynx or esophagus was not considered despite the showing of red blood when removing the esophageal stethoscope and performing intraoral suction, and considering the patient’s coughing out a small amount of blood. An early laryngoscopy was not carried out because a mere intraoral injury was considered responsible. Injury of the pharynx or upper esophagus should be given extra care, as it can bring about bleeding, which can cause anemia and abnormal vitals as well as complications such as pneumonia and hematosepsis. In the present case, the decrease in blood pressure and decreased levels of hemoglobin occurred following surgery, blood transfusion was performed and vasopressors were administered. The continuous bleeding from oropharyngeal injury caused bad conditions of the patient. On the other hand intraoperative fluid administration was less when you consider the amount of blood loss, which could be resulted dehydrated and low blood pressure status of patient. Since then, rapid administration of fluids might have been reduced hemoglobin is measured.
While an iatrogenic injury of the airway under anesthesia is sometimes difficult to avoid, in most cases these injuries can be avoided or mediated by predicting and preparing for possible injuries, or with adept skills. When such an injury occurs, close observation and timely and adequate treatment can minimize discomfort to the patient. The first step of minimizing the risk of airway injury is a pre-surgical examination. Before performing any operating on the airway, a pre-surgical examination of injuries to the teeth, the oral cavity, the vocal cords and the pharynx must be performed [1].
An esophageal stethoscope is relatively less invasive than other monitoring devices used under anesthesia, and it can measure core temperatures with relatively high accuracy in real time. This tool is especially useful in surgeries under general anesthesia in which auscultation is challenging because the distance between the surgical site and the thorax is short, as it makes the patient’s heart and pulmonary sound audible. Furthermore, the esophageal stethoscope is becoming increasingly beneficial, as it can be used for nasotracheal intubation or inserting nasogastric tube [10,11]. However, there are also reports of various side effects caused by an esophageal stethoscope, as its use has become more widespread. The most frequent side effect is the insertion of the esophageal stethoscope into the trachea. It is often quickly diagnosed by air leakage but is sometimes found when incising the airway or when there is trouble pulling out the esophageal stethoscope after the operation [3,6]. Other side effects include the esophageal stethoscope’s blocking the broncus and thus causing hypoxia [7], an incision made to the stethoscope-inserted esophagus after mistaking it for a catheter‐inserted internal jugular vein [3], and pressuring the recurrent laryngeal nerve on the posterior part of cricoid cartilage leading to vocal cord paralysis [8]. To prevent such side effects, the correct use of the esophageal stethoscope must be learned, as follows. When inserting the esophageal stethoscope, the patient’s bleeding tendency must be examined in advance to prevent injury to the esophagus and the pharynx. Moreover, one should be sure to apply enough lubricant onto the tip of the esophageal stethoscope, and the tracheal tube’s balloon should be inflated sufficiently before the slow insertion of the stethoscope. However, because an inflated balloon does not guarantee that the stethoscope is not inserted into the trachea, its mobility forward and backwards must be checked. Unless the stethoscope is inserted into the trachea, caught between the wall of trachea and the balloon of the tracheal tube, it should be able to move back and forth without much resistance [6]. To prevent inflammation on the posterior part of cricoid cartilage, the ideal placement of the esophageal stethoscope is the back of the pyriform sinus of the laryngopharynx [8]. However, that ideal condition is difficult to realize, as the esophageal stethoscope is often relocated inside the esophagus in order to hear the heart and breathing sound at different locations. When the esophageal stethoscope is not inserting easily, one may raise the patient’s jaw or tilt the head such that the path from the mouth to the esophagus is nearly straight. However, the esophageal stethoscope should not be forced in if it is hard to insert despite such methods. Moreover, a tracheal tube that is already inserted should not be moved when inserting the stethoscope.
Generally, the best location of the esophageal stethoscope in adults is 28 to 32 cm into the mouth to hear and monitor the heart and pulmonary sounds effectively [4].
Inserting the esophageal stethoscope in the correct way does not completely eliminate the possibility of side effects. In the event of side effects, quick diagnosis and adequate treatment must be made to minimize discomfort to the patient. In order to do so, the patient’s oral condition and other symptoms should be closely monitored immediately after the insertion of the esophageal stethoscope as well as, after the operation and during the recovery process. Laboratory tests such as a complete blood count test should be conducted. Moreover, one should actively seek to examine the patient as needed if he or she shows a tendency to bleed or shows dubious symptoms under anesthesia, even if the patient is undergoing post-surgical treatments in surgical department.