Acute normovolemic hemodilution for a patient with secondary polycythemia undergoing aortic valve replacement due to severe aortic stenosis - A case report -
Article information
Abstract
Background
A high hematocrit level in patients with erythrocytosis is linked with increased blood viscosity and increased risk of thromboembolism. Therefore, it is necessary to adequately lower the hematocrit level before performing a high-risk surgery.
Case
A 67-year-old male was scheduled for aortic valve replacement due to severe aortic stenosis. The preoperative hematocrit level of this patient was very high due to secondary polycythemia by hypoxia. We decided to perform acute normovolemic hemodilution after anesthetic induction to reduce the risk of thromboembolism in the patient. The patient was discharged after a successful surgery and a post-operative period without any side effects.
Conclusions
We estimate that patients with secondary polycythemia may benefit from acute normovolemic hemodilution to reduce their hematocrit levels while undergoing cardiac surgery using cardiopulmonary bypass. However, it is necessary to control the hematocrit level, since a significant decrease can cause side effects.
Polycythemia, a disease state in which the hematocrit level is higher than normal, is associated with increased blood viscosity that increases the risk for thromboembolism. Specifically, caution is required with cardiopulmonary bypass via thoracotomy, as it reinforces the risk for this disease state [1]. According to Cundy [2], there are 3 intraoperative management procedures that can be employed on patients with polycythemia. First, compression stockings can be used in conjunction with low-dose heparin to prevent thromboembolism during surgery. Second, phlebotomy can be performed as a management procedure once a week prior to surgery to lower the hematocrit level to below 45% as a means to significantly reduce the blood viscosity. The blood drawn during this procedure can be stored for future use, such as in the case of a massive hemorrhage. Last, intraoperative hemodilution is performed by drawing a specific amount of blood and replacing the same amount with colloid solution. This last procedure is noteworthy, as it provides an immediate response to changes in a patient’s physiological status and rapid recovery of normal hematocrit levels during surgery.
Surgeries for patients with polycythemia require a collaboration between the operating surgeon, oncologist or hematologist, and anesthesiologist. While patients with a considerable preoperative period prior to surgery can be considered for radical treatment options to target the root cause of the disease, other patients require phlebotomy to lower their hematocrit level prior to surgery or hemodilution on the day of their scheduled surgery. However, acute normovolemic hemodilution under anesthesia can be performed on patients that cannot undergo phlebotomy or that require immediate treatment, such as emergency surgery [3]. Herein, we report a case of acute normovolemic hemodilution under anesthesia that was performed on a patient scheduled for sutureless aortic valve replacement to treat severe aortic stenosis with secondary polycythemia in order to prevent thromboembolism. We also discuss the effectiveness of the procedure based on previous case studies. We obtained informed consent from the patient.
CASE REPORT
Our patient, a 67-year-old male weighing 66.8 kg and measuring 169.4 cm in height, was scheduled for sutureless aortic valve replacement to treat severe aortic stenosis. He was diagnosed and treated for pulmonary tuberculosis 25 years ago, which resulted in severe sequelae in both lungs, and also underwent surgical resection 14 years prior to treat abdominal liposarcoma. Additionally, our patient was hospitalized for treatment of atrial fibrillation and cardiac insufficiency 7 years ago and diagnosed with hypertension, diabetes, and chronic obstructive pulmonary disease 4 years ago. He was prescribed the following medications: rivaroxaban, digoxin, angiotensin receptor blocker, calcium channel blocker, and an oral hypoglycemic agent. However, rivaroxaban was interrupted 3 days before surgery.
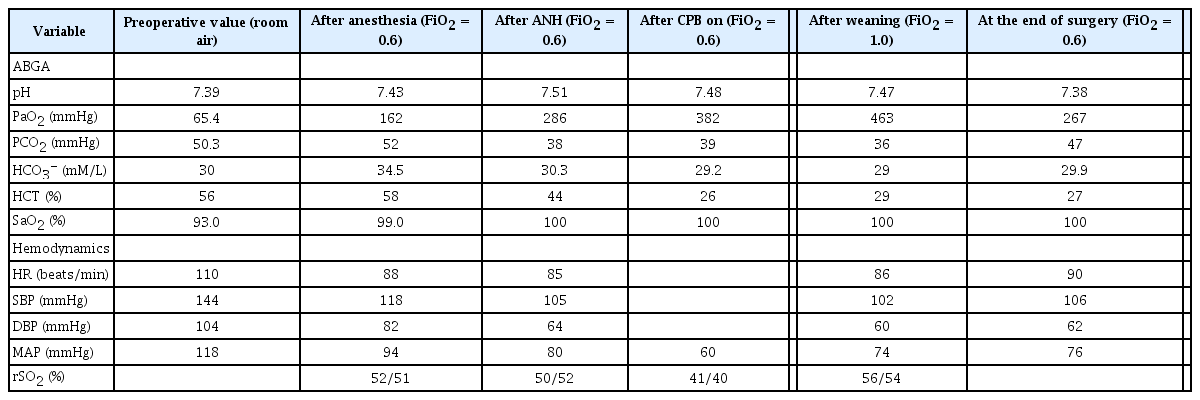
The patient exhibited atrial fibrillation, premature ventricular contraction, left anterior fascicular block, and anterior infarction from electrocardiogram (ECG), with an aortic valve blood flow velocity of 4.3 m/s, mean pressure of 46 mmHg, and aortic valve surface area of 0.7 cm2 from echocardiography. The results indicated severe aortic stenosis, overall decrease in cardiac activity, moderate right ventricular dysfunction, moderate resting pulmonary hypertension, in conjunction with bilateral atrial extension, nonvalvular atrial fibrillation and left ventricular systolic dysfunction. The patient also presented a left ventricular ejection fraction of 17%. A normal result was obtained with coronary arteriography, which is used to identify coronary artery disease. A chest radiography showed pleural effusion in the right lung, an atypical pulmonary nodule, lung parenchymal distortion, bilateral upper lobe pleural thickening, and emphysema, while a pulmonary function test revealed FEV1/FVC was 40%, FEV1 was 27%, FVE was 47%, and TLC was 68%, indicating mixed respiratory failure. A blood test showed 18.5 g/dl of hemoglobin and 57.3% of hematocrit (Table 1), with normal leukocyte and thrombocyte levels. In order to identify the cause of polycythemia, the patient tested negative for janus kinase 2 (JAK2) V617F mutation, and the result of a plasma erythropoietin test was 20.4 IU/L. A bone marrow biopsy was not performed due to patient refusal. However, arterial blood gas analysis during room air breathing was PaO2 of 65.4 mmHg, PCO2 of 50.3 mmHg, and SpO2 of 93% (Table 2), suggesting that the patient may have experienced a secondary polycythemia induced by hypoxia from underlying disease rather than bone marrow disease, in which elevated hemoglobin level could increase the risk of thromboembolism. Thus, after consultation with a thoracic surgeon, the decision was made for the patient to undergo acute normovolemic hemodilution under anesthesia.
Five-lead ECG, pulse oxygen saturation (pulse oximetry), non-invasive blood pressure, arterial pressure from left ulnar artery, bispectral index, and cerebral oxygen saturation were all measured as part of physiological monitoring in the operating room. The patient had a blood pressure of 144/104 mmHg, heart rate of 110 beats/min, and oxygen saturation of 90%. Endotracheal intubation was performed after the patient was anesthetized with administration of 2% lidocaine (60 mg), etomidate (12 mg), rocuronium (100 mg), and fentanyl (150 μg); subsequently, a maintenance dose of midazolam (5 mg), fentanyl (100 μg), and vecuronium bromide (5 mg) were also administered. Arterial pressure was monitored using a left femoral arterial catheter, pulmonary artery catheter (Swan-Ganz catheter, Edward Lifescience, USA), and a central vein catheter, in which the cannulation to the internal jugular vein was conducted using ultrasound guidance. Transesophageal echocardiography was set up for intraoperative assessment of cardiac function and to assess the condition of the heart valves.
Nine hundred and sixty millimeters of blood was drawn from the central vein catheter at a rate of 50 ml/min for 20 min, while injecting 960 ml of 6% hydroxyethyl starch (HES, Volulyte®, Fresenius Kabi Deutchland GmbH, Germany), as the intra-operative arterial blood gas analysis revealed 19.7 g/dl of hemoglobin and 58% of hematocrit. After acute normovolemic hemodilution, the patient’s blood pressure (118/82 mmHg) and heart rate (88 beats/min), as well as hemoglobin (15 g/dl) and hematocrit (44%) were measured. Test results of ROTEM® (Rotational Thromboelastometry, Pentapharm GmbH, Germany) indicated that both EXTEM and FIBTEM were within the normal range (Table 3). The test to determine the infection, acid-fast bacilli smear microscopy, and a culture test could not be completed before the surgery; a blood collected from the patient was disposed of because pleural effusion was observed only on the right side indicating the possibility of infection.
Extracorporeal circulation was initiated 25 min after the start of the surgery, and was sustained for 75 min, during which the hematocrit was maintained between 25 and 30% (Table 2). After weaning from extracorporeal circulation, the patient’s vital signs were stable and heart valve locations and function, as well as the overall cardiac function, were normal based on transesophageal echocardiography. The arterial blood gas analysis results were 9.9 g/dl of hemoglobin and 29% of hematocrit; the ROTEM® test resulted in 101 seconds of clotting time (CT) in EXTEM, which was elongated to 104 s in FIBTEM and 41 mm of amplitude 10 min after the start of the assay (A10) in EXTEM, and was reduced to 6 mm in FIBTEM, indicating a minor decrease in the fibrinogen level [4]. Two units of packed red blood cells, 3 units of fresh frozen plasma, and 8 units of platelets were transfused after consulting with the operating surgeon, due to the fact that the patient showed a bleeding tendency after weaning from extracorporeal circulation. Cerebral oxygen saturation during surgery measured 52/51, which was maintained after acute normovolemic hemodilution but reduced to 41/40 during extracorporeal circulation. Cerebral oxygen saturation eventually recovered to 56/54 after weaning from extracorporeal circulation. The patient was transported to the intensive care unit in a sedated state with endotracheal intubation, which was removed 4 days after admission to the intensive care unit and was then transferred to the general ward the following day. The patient was discharged 15 days after the surgery without further complications. Meanwhile, the patient was diagnosed with secondary polycythemia without further testing, as the blood test results acquired over 2 months post-operatively showed hemoglobin and hematocrit to be lower than normal (Table 1).
DISCUSSION
Polycythemia is a condition where the red blood cell count is higher than normal and can be categorized as polycythemia vera and secondary polycythemia. The 2017 World Health Organization diagnostic criteria describes polycythemia vera as a disease in which the red blood cell count increases due to disorders regarding red blood cell production and provide 3 major and 1 minor diagnostic criteria. The first major criterion is hemoglobin greater than 16.5 g/dl in men and 16.0 g/dl in women, hematocrit greater than 49% in men and 48% in women, or red blood cell mass greater than 25% above the mean predicted value. The second major criterion consists of a bone marrow biopsy indicating hypercellularity through prominent erythroid, granulocytic, and megakaryocytic proliferation, with polymorphic mature megakaryocytic cells of varying sizes. The third major criterion is presence of JAK2 V617F or JAK2 exon 12 mutation and the 1 minor criterion is serum erythropoietin level lower than normal [5]. A diagnosis of polycythemia vera requires that the patient exhibit all 3 major criteria or the first 2 major criteria and the 1 minor criterion. Secondary polycythemia, defined as an increase in red blood cell mass due to elevated serum erythropoietin level, arises as a compensatory mechanism for chronic hypoxia from chronic lung diseases, obstructive sleep apnea, congenital heart diseases, and smoking or, in rare cases, from erythropoietin production from an erythropoietin-releasing tumor, such as those that develop with hepatocellular and renal carcinoma, among others. Our patient underwent surgery without a preoperative assessment, as he refused a bone marrow biopsy or testing for the JAK2 exon 12 mutations, which are required for accurate diagnosis. No further tests, including bone marrow biopsy, were performed, as hemoglobin levels recorded for more than 2 months post-operatively were within the normal range (Table 1).
The main purpose of polycythemia treatment is to prevent thromboembolism, which induces cardiocerebrovascular complications. For this reason, the hematocrit level is maintained below 45% via phlebotomy [2] and cell reduction therapy, such as hydroxyurea, which are also used in patients over 60 years of age or those who have a history of thrombosis. However, the correlation between a high hematocrit level and increased incidence of thromboembolism in secondary polycythemia is controversial. According to the literature, Braekkan et al. [6] reported the risk of venous thromboembolism to be proportional to hematocrit level, and Ristić et al. [7] described polycythemia to be the single most significant risk factor for pulmonary embolism in chronic hypoxemic patients. However, Nadeem et al. [8] reported that secondary polycythemia alone might not signify increased risk for venous thromboembolism.
In a clinical setting, while secondary polycythemia caused by hypoxia from chronic lung diseases and cyanotic heart diseases is treated with phlebotomy, cases related to smoking, erythropoietin-releasing tumor, and anabolic steroid injections are not treated with phlebotomy [9]. Although our patient was a good fit for phlebotomy, as he was elderly and had hypertension, diabetes and atrial fibrillation, which are associated with an increased risk for thromboembolism, phlebotomy was not carried out, as the patient could not complete the treatment for the duration required to reach the target level (once a week for 6 weeks) [10]. Due to severe aortic stenosis characterized by a low left ventricular ejection fraction (17%), accelerated phlebotomy, in which the procedure is carried out twice a week, was deemed unsafe, due to the risk of sharp deterioration in the patient’s condition. After consultation with the operating surgeon, it was decided that the patient would undergo acute normovolemic hemodilution under anesthesia.
Acute normovolemic hemodilution involves collecting a specific amount of blood, based on the target hematocrit, and injecting the same amount of colloid solution, and it is mainly used in patients with high risk for massive hemorrhage so that the blood drawn during the procedure can be stored for self-transfusion to prevent side effects of allogenic blood transfusion. High-risk emergency surgeries for patients with severe polycythemia, such as coronary artery bypass graft using an extracorporeal circulator, uses acute normovolemic hemodilution to prevent side effects of abnormal hematocrit [3]. Acute normovolemic hemodilution in patients with polycythemia causes increased cardiac output and tissue oxygen extraction fraction, as well as decreased systemic vascular resistance as a result of blood dilution, which reduces blood viscosity. A reduced increase in cardiac output from acute normovolemic hemodilution in patients under anesthesia can be attributed to the effect of the anesthetic on the autonomic nervous system and cardiovascular system, which can be manifested as a suppression of change in heart rate and cardiac output increase only contributing to stroke volume increase [11]. Therefore, the absence of heart rate increase under anesthesia makes this a good fit for patients with severe aortic stenosis. Also, acute normovolemic hemodilution under anesthesia allows for an effective response to change in the patient’s physiological status compared to phlebotomy or acute normovolemic hemodilution in an awake state.
The target hematocrit of acute normovolemic hemodilution is determined based on the patient’s condition and the type of surgery, as no specific target exists. In a patient with severe aortic stenosis, accompanied by left ventricular hypertrophy who is undergoing aortic valve replacement, lowering hematocrit to 28% via acute normovolemic hemodilution optimized oxygen transport, and metabolic demand and stimulated postoperative erythropoietin release by decreasing blood viscosity which, in turn, reduced myocardial injury that can cause cardiac complications [12]. However, patients undergoing cardiac surgery under cardiopulmonary bypass need additional caution with hemodilution, as hematocrit below 22% could lead to postoperative complications, such as stroke, myocardial infarction, cardiac arrest, renal failure, and pulmonary edema [13]. We set the target hematocrit level to 45%, considering the patient’s underlying diseases and red blood cell loss during the surgery, and the exchangeable blood volume (V) of 960 ml was calculated using the Bourke and Smith formula V = EBV × ln(HCT0/HCTt) (HCT0: initial hematocrit, HCTt: target hematocrit, EBV: estimated blood volume [0.417 × height + 0.045 × weight – 0.03, 3.8 L for this patient]) [14]. There was no significant change in blood pressure, heart rate, and regional cerebral oxygen saturation from acute normovolemic hemodilution (Table 2). Previous work showed a sharp decline in regional cerebral oxygen saturation when hematocrit reached 30% with acute normovolemic hemodilution [15]. The patient in this case report also exhibited 20% decrease in regional cerebral oxygen saturation when hematocrit became lower than 30% during extracorporeal circulation.
Currently, the target hematocrit level that should be maintained while the patient is getting extracorporeal circulation is unknown. In the past, hematocrit level below 20% during extracorporeal circulation was thought to minimize microcirculation impairment and improve oxygen transport and tissue perfusion. However, recent findings show that a hematocrit level below 22% can increase the perioperative prevalence rate of myocardial infarction, hospitalization period, and cost, as well as the likelihood of patients requiring a blood transfusion [13]. In addition, excessive hemodilution during extracorporeal circulation is shown to cause a sharp decline in regional cerebral oxygen saturation [15]. Therefore, the target hematocrit in this case was set to 25–30%.
Acute normovolemic hemodilution can be a good alternative for patients presenting severe aortic stenosis with secondary polycythemia and undergoing valve replacement that are not eligible for phlebotomy due to time constraints or their physiological conditions. Nonetheless, as aforementioned, an excessively low hematocrit level during extracorporeal circulation can lead to several complications and, thus, requires careful management. Further work is needed to identify the optimal hematocrit range.
SUPPLEMENTARY MATERIALS
Supplementary data containing Korean version of this article is available at https://doi.org/10.17085/apm.2020.15.2.181
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: Ilsang Han, A-ran Lee. Data acquisition: Ho June Kang. Supervision: Young Woo Cho. Writing—original draft: Ilsang Han, Min Gi An. Writing—review & editing: Soon Eun Park, A-ran Lee.