 |
 |
- Search
| Anesth Pain Med > Volume 18(4); 2023 > Article |
|
Abstract
Background
Cerebrospinal fluid (CSF) leakage may cause intracranial hypotension and postural headache. Secondary intracranial hypotension may result from an iatrogenic dural puncture or traumatic injury associated with pain procedures.
Case
A 45-year-old male developed a headache 26 days after spinal pain procedure. Headache was characterized as postural, worsening with standing or sitting and improving while lying down. The pain did not resolve despite the administration of oral and intravenous analgesics. A spinal magnetic resonance imaging revealed epidural venous congestion and a suspicious CSF leak around the left L4/5 level. The patient received an epidural blood patch (EBP), the headache improved dramatically, and the patient was discharged.
When a patient presents with an abrupt onset of severe headache, physicians must consider several possible causes, including emergencies. However, if a patient has a characteristic postural headache that is relieved by lying down and worsened by sitting or standing, a cerebrospinal fluid (CSF) leak may be the cause. Intracranial hypotension occurs when leakage from the epidural space reduces the volume of intracranial CSF. CSF leakage can be spontaneous or secondary. Secondary intracranial hypotension is caused by iatrogenic CSF leakage following lumbar puncture, surgery, or dural rupture due to spinal trauma. Secondary CSF leakage typically manifests at a specific site corresponding to the needle entry point. However, spontaneous intracranial hypotension (SIH) occurs in structurally vulnerable areas of the dura mater, often involving multiple sites simultaneously [1].
The possibility of dural puncture has been mentioned in the literature for spinal pain procedures such as transforaminal epidural block, dorsal root ganglion block, selective nerve root block, and posterior medial branch block. However, we were able to find only one report of post-dural puncture headache (PDPH) a few hours after a cervical medial branch block [2]. Most cases of PDPH occur within 72 h after dural puncture, while some cases of PDPH may occur up to 2 weeks later [3,4]. However, no cases of later onset have yet been reported.
Generally, in patients with severe complaints of postural headache due to intracranial hypotension, an epidural blood patch (EBP) is an effective management strategy for CSF leakage [5]. Both primary and secondary intracranial hypotensions can be effectively treated using EBP. Therefore, rapid diagnosis and treatment are essential for relieving patient discomfort.
Here, we report our experience with the evaluation and treatment of abrupt-onset postural headache in a male who underwent a spinal pain procedure for lumbar disc herniation 26 days before symptom onset, along with a review of the literature. To the best of our knowledge, this is the case with the most delayed onset.
Written informed consent was obtained from the patient before publication of this case report.
A 45-year-old male (height, 168 cm; weight, 69 kg) presented to our neurology outpatient clinic complaining of an unknown-onset headache without a history of trauma. The headaches began in the morning. The pain, rated as 5 on the visual analogue scale (VAS), was in the fronto-occipital region and radiated to the posterior neck. Headache was described as a continuous dull ache, relieved by lying down and aggravated by sitting or standing. The patient experienced nausea and vomiting. Neurological examination revealed no focal neurological deficits. The subsequent laboratory tests revealed no significant abnormalities. Computed tomography (CT) of the brain was performed to rule out cerebral hemorrhage, which revealed no significant abnormalities. With no specific findings, the patient was discharged and prescribed naproxen.
The following day, the patient presented to the emergency department with a worsening headache. The VAS score had increased to 7. A repeat brain CT scan was recommended; however, the patient declined further evaluation because he had an outpatient neurology appointment scheduled the next day and was discharged from the emergency department after receiving tramadol 50 mg intravenously.
The next day, the patient was admitted to the neurology outpatient department for reassessment and treatment of headache. A detailed post-admission questionnaire confirmed the patientŌĆÖs history of lumbar disc herniation (Fig. 1) and that he had undergone repeated nerve block at a local pain clinic. In particular, he had last undergone a spinal pain procedure at the clinic 26 days before the onset of his headaches; he reported that it was uneventful and that he had no headaches or other abnormal symptoms after the procedure. However, it is unclear which spinal pain procedure the patient underwent. Based on the patient's statement that he received a single injection under C-arm guidance for the painful area (left) and the clinical context, it was assumed that he received a transforaminal nerve root block at the lumbar level. Laboratory tests performed after admission showed no abnormalities in C-reactive protein levels or white blood cell counts. For conservative management of the patient's headaches, bed rest and hydration with 0.9% saline 80 ml/h and oral acetaminophen/tramadol were prescribed.
However, the pain persisted, and the patient was referred to our pain clinic for further evaluation and management. Considering the patient's injection history and characteristic postural headache simultaneously, the possibility of PDPH was raised; however, given that the interval between the injection treatment and the patient's headache was 26 days, we considered the likelihood of a direct association between the procedure and headache to be low. Therefore, we initially considered the possibility of SIH and, performed a bilateral greater occipital nerve blockade, which is a good therapeutic alternative to improve headache resulting from SIH [6]. The patient was referred for a spinal magnetic resonance imaging (MRI), and the need for EBP for persistent symptoms was explained to him. In the evening, the patient underwent MRI of the entire spine, which revealed extensive thickening and enhancement of the epidural space extending from the cervical spine to the sacrum, with concomitant engorgement of the epidural veins (Fig. 2) and a CSF leak in the left lateral recess at the L4/5 level around the L5 nerve root (Fig. 3). The results showed a CSF leak at the left L4/5 level (Fig. 4).
The following day, the patient underwent EBP at the pain clinic after providing informed consent for the procedure. Two sterile fields were created for the two operators, one for the blood draw and the other for the epidural procedure. An epidural needle was inserted at L4/5 interspinous space using the loss of resistance to saline to identify the epidural space. A syringe containing autologous venous blood was drawn by a second operator using aseptic techniques. Finally, 15 ml of autologous blood was slowly injected through the epidural needle. Immediate and complete symptomatic improvements were observed after EBP. The postural headache improved from a VAS score of 7 to 0, and the patient was discharged the following day. The patient showed complete improvement with no headache at subsequent outpatient visits after 2 weeks
The International Classification of Headache Disorders (ICHD-3) subdivides headaches caused by low CSF pressure into PDPH, CSF fistula headaches, and headaches attributed to SIH (Table 1) [7]. According to the ICHD-3 commentary on headaches attributed to low CSF pressure, evidence of causation may depend on the temporal relationship of the onset to the presumed cause and the exclusion of other diagnoses. Therefore, patients with suspected CSF leaks often undergo spinal MRI to locate the suspected leak site. The imaging features of CSF leaks on spinal MRI include epidural fluid collection, dural sac collapse, and engorgement of the epidural venous plexus. Imaging studies showed evidence of CSF leakage in this patient (Figs. 2-4).
In this regard, the patientŌĆÖs clinical presentation was consistent with a headache caused by low CSF pressure. First, the headache was postural. Intracranial hypotension occurs when the volume of CSF decreases owing to leakage, causing the brain to sag in the cranial vault. This sagging causes traction on the sensory nerves of the meninges and bridging veins, resulting in a headache [8]. The traction on the meninges is increased in an upright position, leading to an increase in the intensity of the headache when standing; thus, creating a postural headache. Second, the patient experienced immediate and definite relief from the headache after EBP. Although various treatments, such as bed rest, analgesics, nonsteroidal anti-inflammatory drugs, hydration, intravenous caffeine, and caffeine-containing products have been used as they usually provide only temporary pain relief. The definitive and most effective treatment option, with an approximate success rate of 85%, is the application of EBP, especially a targeted EBP [5,9]. Thirdly, the patient underwent a spinal pain procedure that was supposed to be a transforaminal epidural nerve root block, a procedure that may have inadvertently included a dural puncture. During the course of a nerve root block, the needle may access the dura near the nerve root, and a dural puncture with CSF leakage may occur. All of these are equivalent to a headache attributed to low CSF pressure in the ICHD-3 classification.
However, the clinical presentations and imaging findings in these patients are not consistent with SIH. According to the diagnostic criteria of headache attributed to SIH in ICHD-3 (Table 1), SIH must not have been caused by a procedure or trauma known to cause CSF leakage. It cannot be diagnosed in patients who underwent a dural puncture within the previous month. SIH occurs due to three main sources of CSF leakage in the absence of a clear history of neuraxial procedures. These main sources are dural tears, typically associated with calcified protrusions of the thoracic intervertebral disc (type 1), rupture of a meningeal diverticulum (type 2), and development of a CSF-venous fistula (type 3) [10]. The incidence of SIH is estimated to be two to five per 100,000 population, with a higher prevalence in females than males, being approximately twice as common. The peak age of onset is typically around 40 years [1,11]. Approximately two-thirds of people with spontaneous CSF leaks have evidence of connective tissue disease [1]. Therefore, this case does not meet the diagnostic criteria for SIH.
We can also consider the possibility of PDPH. According to ICHD-3, PDPH occurs within 5 days of lumbar puncture and is caused by CSF leakage through dural puncture. This patient had a history of pain procedure at the lumbar area, suggesting a possibility of a dural puncture caused by the advancing needle. However, no definitive evidence of a puncture was found. The absence of complications during the procedure and the lack of immediate post-procedural headaches reported by the patient made it challenging to confirm the presence of PDPH. Early- or delayed-onset PDPH occurs mostly 1-7 days after dural puncture, and one case has been reported after 12 days in a 33-year-old female who received an epidural for vaginal delivery [12]. Delayed-onset PDPH is rare, and the exact underlying mechanisms are not fully understood; however, it is associated with slower leakage of CSF through the dural hole, resulting in a gradual decrease in CSF volume and subsequent onset of symptoms.
In this case, because he developed a headache 26 days later, we hypothesized the following: At the time of the procedure, a small hole was created when the needle grazed the dura, where the CSF leak may have become severe enough to cause hypotension for 26 days. Another possibility is that the part of the dura that was weakened by needle grazing was torn by a minor event that increased the CSF pressure, such as coughing or sneezing [13]. Therefore, based on the patient's symptoms, MRI findings, and history of spinal pain procedure, our impression of the patient's headache was secondary intracranial hypotension, which was considered highly correlated with the pain intervention.
In addition to the spinal or epidural anesthesia settings, where a deliberate dural puncture is performed, spinal pain procedure performed near the dura mater can also result in secondary intracranial hypotension. We should remember that problems can occur even long after the procedure, as in this case. If this type of headache is suspected, a quick examination, including MRI, can identify the area of CSF leakage, and immediate application of EBP can reduce unnecessary pain and discomfort for the patient.
Notes
Fig.┬Ā1.
T2W sagittal and axial lumbar MRI shows (A) disc bulging at the L4/5 level (red arrow) and (B) compression of Lt L5 nerve roots (red arrow). MRI: magnetic resonance imaging, Lt: left.
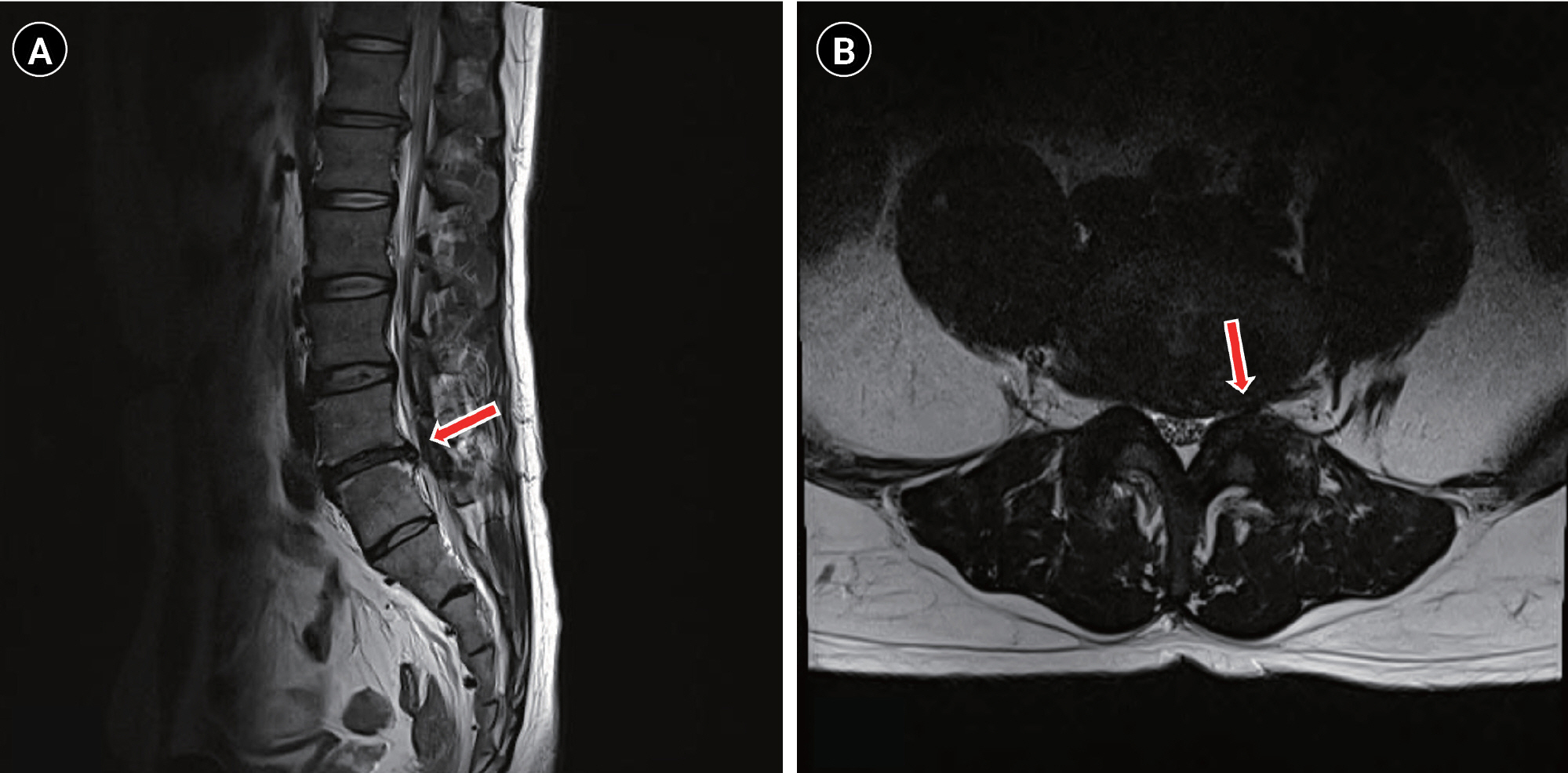
Fig.┬Ā2.
T2W sagittal whole spine MRI shows diffuse thickening and enhancement of the epidural space from the (A) cervical (red arrow), (B) thoracic (red arrow) spine to the sacrum with engorgement of the epidural veins compatible with intracranial hypotension. (C) Fat suppressed contrast-enhanced T1 axial MRI shows dorsal enhancement of the epidural space (red arrow). MRI: magnetic resonance imaging.
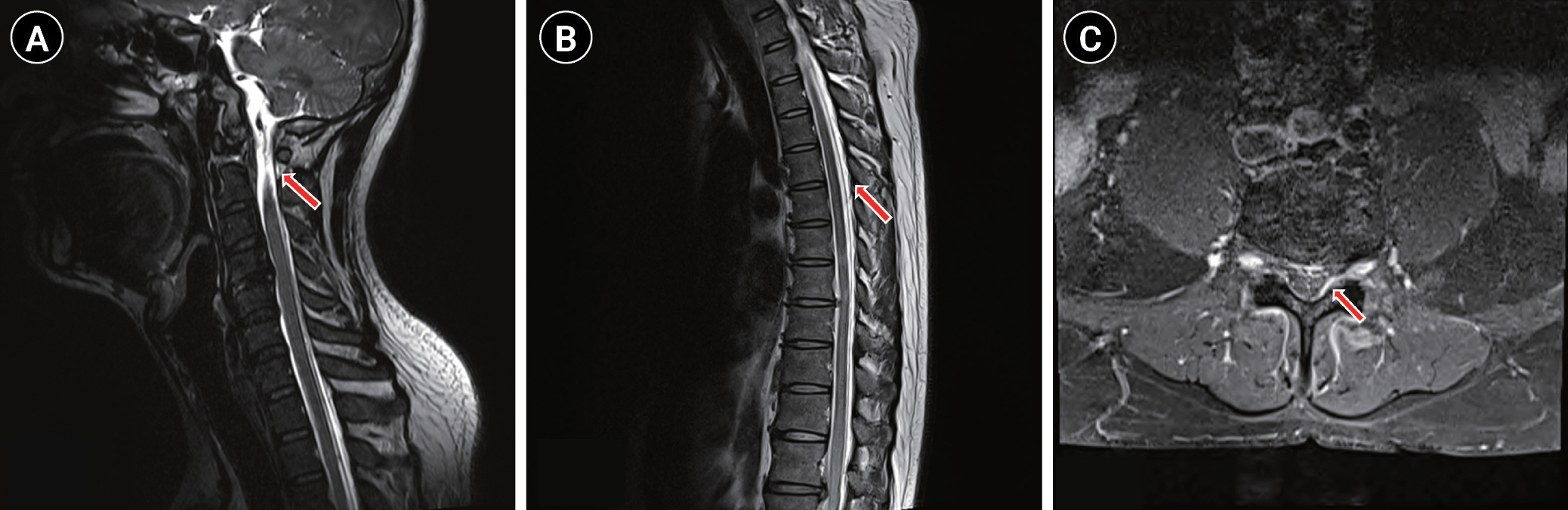
Fig.┬Ā3.
Contrast-enhanced T1W axial spine MRI shows enhancement in the left lateral recess at the L4/5 level near L5 nerve root, suggesting CSF leakage (red arrow). MRI: magnetic resonance imaging, CSF: cerebrospinal fluid.
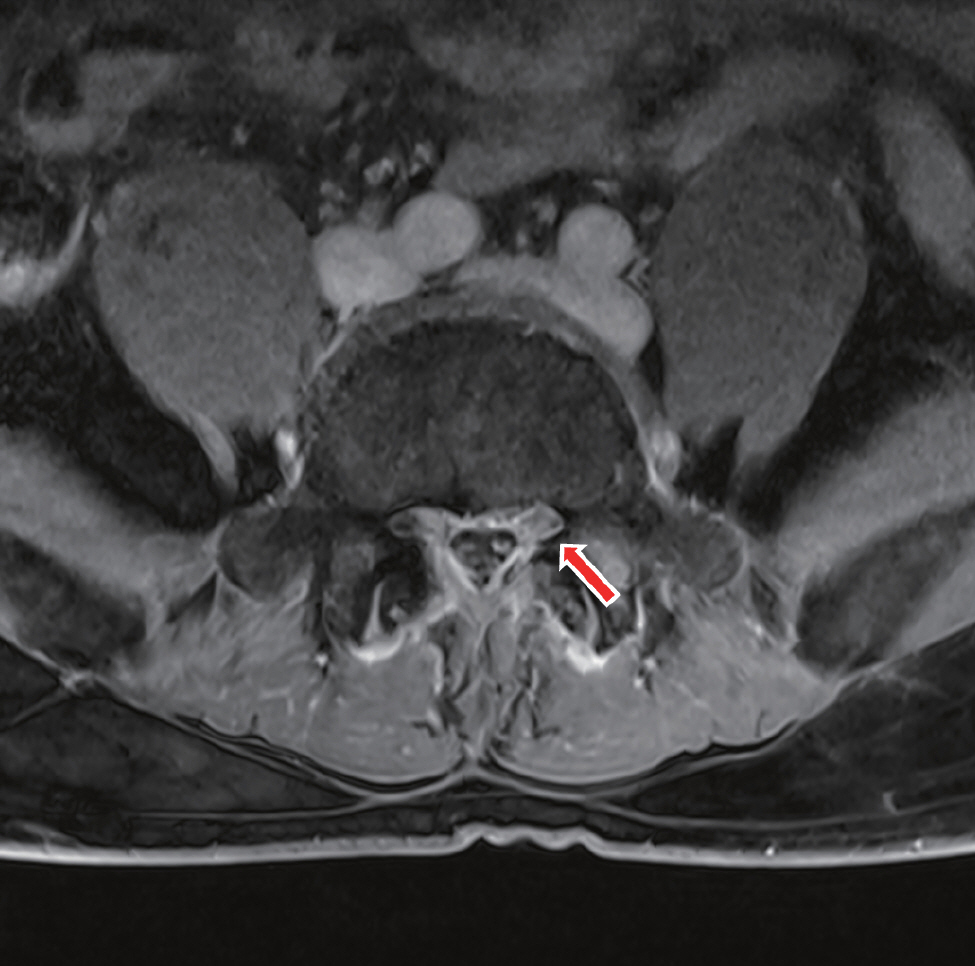
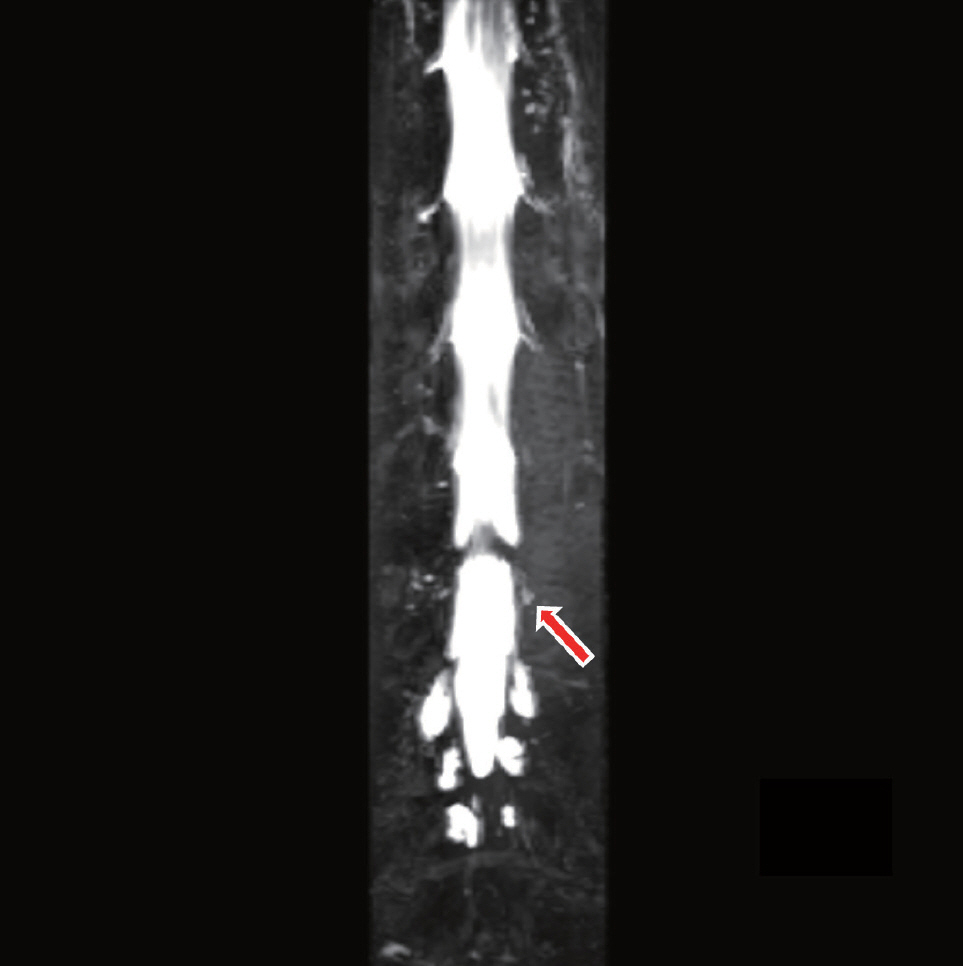
Fig.┬Ā4.
Heavily T2 MR myelogram shows CSF leakage into the left posterior spinal epidural space at L4-5 (red arrow). MR: magnetic resonance, CSF: cerebrospinal fluid.
Table┬Ā1.
Diagnostic Criteria for Headache Attributed to Low CSF Pressure and Its Subtypes (Modified from ICHD-3)
REFERENCES
1. Rajpal S, Nambiar M, Castanelli D, Khabaza A, Asadi H, Jhamb A, et al. Spontaneous intracranial hypotension and spinal epidural CSF leaks: diagnosis and management. J Clin Neurosci 2023; 111: 46-56.


2. Lee YI, Soh HJ, Kim ED. Postdural puncture headache after cervical medial branch block. Soonchunhyang Med Sci 2018; 24: 196-8.


3. Vilming ST, Schrader H, Mostad I. The significance of age, sex, and cerebrospinal fluid pressure in post-lumbar-puncture headache. Cephalagia 1989; 9: 99-106.

4. Kuntz KM, Kokmen E, Stevens JC, Miller P, Offord KP, Ho MM. Post-lumbar puncture headaches: experience in 501 consecutive procedures. Neurology 1992; 42: 1884-87.


5. D'Antona L, Jaime Merchan MA, Vassiliou A, Watkins LD, Davagnanam I, Toma AK, et al. Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome: a systematic review and meta-analysis. JAMA Neurol 2021; 78: 329-37.


6. Hong JH, Lee HW, Lee YH. Greater occipital nerve blockade using ultrasound guidance for the headache of spontaneous intracranial hypotension: A case report. Anesth Pain Med (Seoul) 2022; 17: 62-6.



7. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 2018; 38: 1-211.


8. Marcelis J, Silberstein SD. Spontaneous low cerebrospinal fluid pressure headache. Headache 1990; 30: 192-6.


9. Seebacher J, Ribeiro V, LeGuillou JL, Lacomblez L, Henry M, Thorman F, et al. Epidural blood patch in the treatment of post dural puncture headache: a double blind study. Headache 1989; 29: 630-2.


10. Dobrocky T, Nicholson P, H├żni L, Mordasini P, Krings T, Brinjikji W, et al. Spontaneous intracranial hypotension: searching for the CSF leak. Lancet Neurol 2022; 21: 369-80.


-
METRICS

-
- 0 Crossref
- 972 View
- 38 Download
- Related articles in Anesth Pain Med
-
Treatment of rocuronium-induced anaphylaxis using sugammadex - A case report -2021 January;16(1)
- ARTICLE & TOPICS
-
- Topics
-
- Neuroscience in anesthesiology and critical care
- Anesthetic Pharmacology
- Obstetric Anesthesia
- Pediatric Anesthesia
- Cardiothoracic and Vascular Anesthesia
- Transplantation Anesthesia
- Spinal Pain
- Regional Anesthesia
- Neuromuscular Physiology and Pharmacology
- Airway Management
- Geriatric anesthesia and Pain
- Others







